Lisa M. Manus, PhD; Carl P. Myers, PhD; Robert D’Ambrogio, BS; Gokul V. Govindaraju, PhD; Guofeng Xu, PhD; Yun-Po Zhang, PhD, DDS (Hon); and James G. Masters, PhD
Abstract: Effective and accessible oral care strategies, like the use of a multi-benefit, antimicrobial toothpaste, are a key tool in preventive public health. For over 30 years, Colgate Total toothpastes have represented a gold standard in multi-benefit toothpastes to help fight bacteria and provide whole-mouth care. This review introduces the next generation of Colgate's research and development featuring stannous fluoride (SnF2) stabilized by nitrate and phosphates. The uniqueness of this engine is detailed through a review of SnF2 oral benefits, the historic challenges associated with SnF2 toothpastes, and the advantages that this chemistry can bring to patients seeking multi-benefit oral care. With this novel technology, a new balance in efficacy, stability, and streamlined design enables flexible formulation and customized user experiences inspired by key therapeutic areas.
The tipping point between oral health and disease is influenced by a variety of inherent and environmental risk factors. The health of the oral cavity can be impacted by genetics, diet, accessibility to routine dental care, perceptions of and past experiences with dental professionals, socioeconomic status, and daily oral hygiene practices.1-4 Despite scientific advances in fundamental knowledge and the development of therapies to help reduce risk factors, caries and periodontal disease remain as two of the most dominant oral health issues globally. Currently, approximately 3.5 billion people around the world have experienced at least one form of these diseases in their lifetime.5,6 Accessible, affordable, and effective interventions that help attenuate the risk of oral diseases are key. Many common oral health conditions are to a great extent preventable and can be reduced through appropriate oral care.7-9In addition to routine dental exams, daily oral hygiene with a fluoride toothpaste featuring an antimicrobial control agent can provide an important action to help prevent the risk of oral diseases.10-12
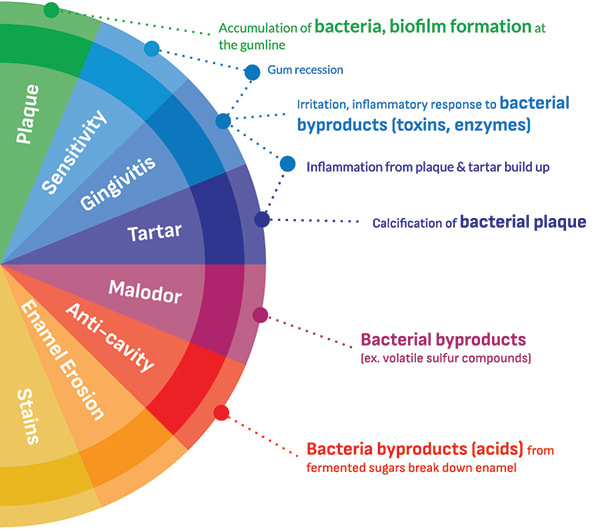
A root cause of many common oral issues is the growth of pathogenic bacteria in the mouth (Figure 1).13 Directly or indirectly, these microorganisms can cause damage to hard and soft tissues in the oral cavity, potentially leading to advanced states of disease over time. Bacteria can be present throughout the mouth in planktonic form or within diverse biofilms anchored to an oral surface like the teeth, tongue, cheeks, and gums. If an oral biofilm is left undisturbed, it grows and matures into a biofilm that can be harmful to one's oral health. These mature oral biofilms serve as a safe haven for bacteria and enable them to exchange nutrients, form communication networks with each other to defend against threats, and build structures to resist stress from the environment.14,15 If these biofilms are not reduced in mass or vitality (and this growth is coupled with additional risk factors favoring disease), they continue to proliferate and a shift in the composition of the microflora may occur that favors pathogenic bacteria.16-18 An abundance of pathogenic bacteria and their byproducts can be a root cause of many common oral health issues given their ability to release toxins that irritate gums, produce volatile sulfur compounds promoting oral malodor, and generate acids that break down dental hard tissues.
To a certain degree, some oral bacteria and biofilms can be removed through mechanical actions such as brushing with a non-antimicrobial fluoride toothpaste and flossing. However, a lack of consistent compliance to a comprehensive oral hygiene routine can limit the ability of these actions to effectively fight bacteria.19 Intervention with antimicrobial agents has been clinically proven to reduce oral bioburden significantly by helping control bacteria growth and mitigate bacterial byproducts, even between brushings.20-22 Antimicrobial agents can also help fight bacteria in areas of the mouth where toothbrushing may be limited, such as interproximally and on soft-tissue surfaces like the buccal mucosa, tongue, and gums. Therefore, targeting bacteria, a root cause of many common oral health issues, with an antimicrobial-based dentifrice provides a more viable route to help in oral disease prevention.
Stannous Fluoride: A Powerful Partner With Challenging Applicability
Stannous fluoride (SnF2) is a well-known active ingredient in dentistry offering multiple oral care benefits.23-28 SnF2 offers both hard tissue and soft tissue benefits due to its multiple modes of action. Sn2+ ions are known to interfere with bacterial metabolic function, slowing their growth and preventing bacterial acid production.29,30 SnF2 has also been shown to form mineral precipitates on dental hard tissues like dentin and enamel.31,32 These precipitates can occlude exposed dentin tubules, which are a major cause of dentin hypersensitivity. As a result of these diverse modes of action, SnF2 dentifrices have shown clinically significant reductions in dental plaque formation, gingivitis, malodor, and hypersensitivity pain in addition to prevention of caries and enamel erosion.28,33,34
While the multiple benefits provided by SnF2 are a clear advantage to other fluoride sources such as sodium fluoride and sodium monofluorophosphate, which only offer caries protection, SnF2 dentifrices are challenging to formulate. While relatively inert as a simple salt, SnF2 dissociates into its constituent ions (F- and Sn2+) in aqueous environments that are common in typical dentifrice formulations.29,35 Aqueous Sn2+ ions are sensitive to air, heat, and water presenting a critical obstacle. Moreover, a dichotomy exists as Sn2+ ions are inherently unstable at the optimal conditions for bioactive fluoride. Fluoride ion stability is best in high-water formulations at near neutral or slightly alkaline pH (pH 7-9).36-38Stannous salts can readily hydrolyze under aqueous conditions, especially above pH 4, resulting in precipitation from solution and/or subsequent oxidation to Sn4+.30,39-41
Because clinical efficacy is dependent on the Sn2+ state of the ion, it is paramount to try to maintain this oxidation state throughout the lifetime of the product.29,42-44 If not strategically designed, a SnF2 dentifrice can run the risk of poor efficacy, surface enamel staining, or a perceivable metallic taste. Even the US Food and Drug Administration (FDA) monograph acknowledges this, stating, "The careful formulation of stannous fluoride dentifrices to prevent rapid oxidation and hydrolysis, and thereby inactivation, of stannous ions is critical for clinical effectiveness of these dentifrices."40 A delicate balance must be achieved between maximizing the long-term chemical stability of the active ions in the toothpaste, their potent bioactivity in the mouth, and delivery in a consumer acceptability product form for continued and consistent use. Historically, several different strategies have been pursued in consumer dentifrices. While these mechanisms help address SnF2 stability and bioactivity, they can impact user acceptability. Taste, mouth feel, and product look can be extremely different in a stabilized SnF2 toothpaste compared to an ordinary sodium fluoride toothpaste, limiting universal adoption and compliance. Anhydrous formulations can be useful to prevent hydrolysis and subsequent oxidation to the Sn4+ species but may compromise consumer experience in mouth feel or taste.29 These toothpastes may also exhibit poor standup on a brush and messy textures resulting from a lack of adequate viscosity-building agents that function in low-water formulations.45 Some products use additional sources of stannous salts, such as stannous chloride, to compensate for Sn2+ ions oxidized or hydrolyzed in the formulation. However, this approach was shown to be inefficient, perpetuating a high level of inactive Sn4+ ions in the toothpaste.29 This method (like anhydrous formulas) can also lead to high-cost manufacturing or ingredients, limiting affordable options for all users. Chelation with ligands such as pyrophosphate, hexametaphosphate, or organic compounds has proven effective at slowing oxidation, likely through steric complexation mechanisms.29,32,46 However, complexation mechanisms of Sn2+ must be carefully executed, as they may introduce solubility limitations or reduce their bioavailability. In rare cases, SnF2 toothpastes with complex stabilization systems and limited water content have been implicated in the development of oral discomfort and other localized oral reactions such as oral mucosal exfoliation (Figure 2).47,48 Given these collective complexities, the consumer products industry devotes significant resources to innovating new ways to optimize a balance of the chemical stability and bioactivity of Sn2+ ions in oral care products. However, these complex SnF2 stabilization mechanisms usually have decreased formula flexibility, limiting major differentiation and innovation between products, such as building in new benefits and/or changes in foam, flavors, and product esthetics.
Colgate TotalSF®: A Breakthrough Stannous Toothpaste Stabilized by Phosphates
In 2019, Colgate TotalSF® (Colgate-Palmolive Co., colgatepalmolive.com) was launched in the United States. This formula was comprised of SnF2 stabilized in a single-phase formulation in the presence of phosphate sources such as tetrasodium pyrophosphate and zinc phosphate in an appropriate organic acid buffer system.29 The toothpaste was formulated at near neutral pH in a high-water (>20%) formulation, helping maintain high levels of fluoride ion stability in the toothpaste matrix. X-ray absorption near edge spectroscopy (XANES) showed the ability of this chelation scheme to maintain higher levels of Sn2+ (both free ions and chelated ions) in this toothpaste formulation when compared to other commercially available SnF2 toothpastes.29 This system was also observed to be highly efficient when normalized to total stannous in the base. Considering the inclusion of only 0.454% SnF2 in the toothpaste, the ratio of Sn2+ to inactive Sn4+ was 6.63; other commercially available SnF2 toothpastes (including those supplemented with additional stannous salts) had significant levels of inactive Sn4+ (40% to 59% of the total stannous in the base) with ratios of less than 1.5.29 Correlation between the Sn2+ and Sn4+ ratios in each product and antibacterial efficacy was observed in vitro.29 Improved stain prevention was also observed with this toothpaste in vitro in assay comparisons to other SnF2 commercial products highlighting the importance of oxidative stability imparted by the formulation scheme.45 Clinical efficacy of this toothpaste has been demonstrated in multiple studies across a broad spectrum of oral health conditions, including reductions in plaque bacteria, gingivitis, and hypersensitivity pain.31,49,50 The mode of action of the formulation has been examined in clinical studies from bacterial as well as the innate functions of the mouth.51,52 Specific bacteria, bacterial gene pathways, and oral inflammatory biomarkers influenced by stannous have been identified, concomitantly resulting in healthy biofilm and reduced gingival inflammation.
Next Generation SnF2: Stannous Stabilized by Nitrate and Phosphates
Recently, researchers discovered that a proprietary combination of phosphates and nitrate ions, two common ingredients in oral care, can help both solubilize and stabilize Sn2+ ions in a revolutionary manner.53 These ingredients appear to work synergistically. Pyrophosphate-chelated Sn2+ ions maintain this chemistry as a water-soluble, bioactive form at near neutral pH. Simultaneously, nitrate appears to help block the chemical reaction pathways that lead to Sn4+ conversion, even under conditions known to promote oxidation, including heat, high-water environments, and dissolved oxygen gas. In simple aqueous solutions, this Sn2+ active engine showed almost nine times more Sn2+ (nearly 90% of starting amount) remaining after 2 weeks at 60°C in comparison to SnF2 alone (<10% of starting amount remaining).53 This system was also shown in vitro to significantly suppress the growth of known oral disease-causing bacteria (Streptococcus mutans and Porphyromonas gingivalis) and reduce production of the pro-inflammatory cytokine interleukin (IL)-8 in P gingivalislipopolysaccharide-challenged cells.53
Performance testing, including clinical studies, of toothpastes formulated with 0.454% SnF2 stabilized by nitrate and phosphates have shown impressive results. Application of this engine in various toothpaste backbones has demonstrated the safety, versatility, and high quality associated with this approach. Many of these results are summarized in this special issue, including:
• significant and clinically relevant reductions of bacteria in saliva and on multiple oral surfaces (including teeth, tongue, cheeks, and gums) 12 hours post-brushing after 4 weeks of continuous use54
• clinical results showing superior reduction of plaque after 3 months of use; powerful gum care with 100% of patients in a clinical study showing improvement in gingival index over 6 months of continuous use55
• significant and clinically relevant reductions in hypersensitivity pain after 1 day (two times brushing) in comparison to a 5% potassium nitrate desensitizing toothpaste56
• significant reductions in oral malodor, with 85.7% of patients achieving organoleptic scores corresponding to pleasant breath overnight 12 hours post-brushing after 3 weeks of continued use.57
With this active engine, the manufacturer is also able to formulate against key consumer needs associated with lifestyle and environment, including clinically proven tooth stain reduction measured after 3 and 6 weeks of product use.58
Finally, this active engine enables an improved pathway to incorporate new benefits and user experiences in a SnF2 toothpaste. Streamlined and discrete, this technology needs only two ingredients at low levels to ensure SnF2 stability in a high-water-content toothpaste at near neutral pH, leading to less complexity in manufacturing, fewer flavor restrictions required to mask unfavorable metallic tastes, reduced risk of teeth staining, and decreased toothpaste discoloration. Furthermore, the simple phosphate chelation mechanism used in this engine not only helps limit interactions with a wide variety of formula excipients but also maintains the bioactivity of this active engine upon brushing. The unique combination provides a new opportunity for flexible formulation of a diverse portfolio of efficacious SnF2-based formulas. In addition to innovation through new benefits and functional ingredients, a wider variety of experiences (foaming profiles, flavors, cooling agents, esthetics) can now be developed to engage and adapt to the diverse preferences of a global population (Figure 3 through Figure 6). In consumer studies, users rated the new toothpaste higher in flavor and foaming attributes (in comparison to the original Colgate TotalSF toothpaste). They also saw the new product as better matched with health-based attributes like "provides long lasting protection for my mouth" and "allows me to be proactive about my oral health."57
Evolving to a New Era
Colgate's bioactive SnF2 engine leveraging nitrate and phosphates stabilization technology offers a novel, streamlined, efficacious approach to SnF2 stability and bioavailability, with distinct advantages, including more efficient manufacturing and enhanced formulation flexibility for broader versatility in flavors, cooling agents, esthetics, and foaming profiles. This novel SnF2 bioactive engine enables a new, expanded people-centered approach focused on the toothbrushing experience while maintaining multi-benefit efficacy against plaque, gingivitis, cavities, calculus, hypersensitivity, enamel erosion, oral malodor, and tooth staining.
Prevention strategies work most effectively with compliance.59,60 This engine has also created a unique, new opportunity given its advantages in flavoring, complexity reductions, and resistance to excipient ingredient interactions. Even in a therapeutic toothpaste, the right flavor, foam, color, mouth feel, texture, and cosmetic benefits help to drive compliance. However, different users can have vastly different perceptions and preferences that manifest as reasons to believe or not believe in a product's performance and continued use. The development, customization, and curation of unique experience profiles befitting different groups better promotes consistent use, enabling as many different groups of users as possible to experience the scientific and clinical benefits of a SnF2 toothpaste.
ACKNOWLEDGMENTS
The author contributions were as follows: LM, CM, RD, and GG: conceptualization, writing original draft; GX: conceptualization, project administration, supervision, resources; YZ: writing, review, and editing; JM: project administration, supervision, resources. All authors contributed to writing, review, and editing.
DISCLOSURES
Research described in this article was funded by Colgate-Palmolive Company. All authors are employees of Colgate-Palmolive Co. CM and GX have patents #US10918580B2 and #US11723846B2 issued to Colgate-Palmolive Co.
DATA AVAILABILITY
The documents containing the results of the research herein described are confidential. The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
About the Authors
Lisa M. Manus, PhD
Director Research and Development (R&D), Colgate-Palmolive Co., Piscataway, New Jersey
Carl P. Myers, PhD
Director R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Robert D'Ambrogio, BS
Senior Principal Scientist R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Gokul V. Govindaraju, PhD
Principal Scientist R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Guofeng Xu, PhD
Senior Director R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Yun-Po Zhang, PhD, DDS (Hon)
Senior Vice President and Distinguished Fellow, Clinical Research, Colgate-Palmolive Co., Piscataway, New Jersey
James G. Masters, PhD
Vice President R&D, Colgate-Palmolive Co., Piscataway, New Jersey
References
1. Northridge ME, Kumar A, Kaur R. Disparities in access to oral health care. Annu Rev Public Health. 2020;41:513-535.
2. Vieira AR, Modesto A, Marazita ML. Caries: review of human genetics research. Caries Res.2014;48(5):491-506.
3. Mueller M, Schorle S, Vach K, et al. Relationship between dental experiences, oral hygiene education and self-reported oral hygiene behaviour. PLoS One. 2022;17(2):e0264306.
4. Hujoel PP, Lingström P. Nutrition, dental caries and periodontal disease: a narrative review. J Clin Periodontol. 2017;44 suppl 18:S79-S84.
5. Elamin A, Ansah JP. Projecting the burden of dental caries and periodontal diseases among the adult population in the United Kingdom using a multi-state population model. Front Public Health.2023;11:1190197.
6. GBD 2017 Oral Disorders Collaborators; Bernabe E, Marcenes W, Hernandez CR, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J Dent Res. 2020;99(4):362-373.
7. World Health Organization. Oral health. WHO website. March 14, 2023. https://www.who.int/news-room/fact-sheets/detail/oral-health. Accessed September 19, 2024.
8. Listl S, Baltussen R, Carrasco-Labra A, et al. Evidence-informed oral health policy making: opportunities and challenges. J Dent Res. 2023;102(12):1293-1302.
9. FDI World Dental Federation. Vision 2030: delivering optimal oral health for all. FDI website. https://www.fdiworlddental.org/vision2030. Accessed September 19, 2024.
10. Teles RP, Teles FRF. Antimicrobial agents used in the control of periodontal biofilms: effective adjuncts to mechanical plaque control? Braz Oral Res. 2009;23 suppl 1:39-48.
11. Rajendiran M, Trivedi HM, Chen D, et al. Recent development of active ingredients in mouthwashes and toothpastes for periodontal diseases. Molecules. 2021;26(7):2001.
12. Spatafora G, Li Y, He X, et al. The evolving microbiome of dental caries. Microorganisms. 2024;12(1):121.
13. Morrison AG, Sarkar S, Umar S, et al. The contribution of the human oral microbiome to oral disease: a review. Microorganisms. 2023;11(2):318.
14. Kolenbrander PE, Andersen RN, Blehert DS, et al. Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66(3):486-505.
15. Jakubovics NS, Goodman SD, Mashburn-Warren L, et al. The dental plaque biofilm matrix. Periodontol 2000. 2021;86(1):32-56.
16. Sanz M, Beighton D, Curtis MA, et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. 2017;44 suppl 18:S5-S11.
17. Mira A, Simon-Soro A, Curtis MA. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J Clin Periodontol. 2017;44 suppl 18:S23-S38.
18. Kilian M, Chapple ILC, Hannig M, et al. The oral microbiome - an update for oral healthcare professionals. Br Dent J. 2016;221(10):657-666.
19. Cummins D, Marsh PD. Changing the paradigm of daily prevention to achieve whole mouth health in the 21st century. J Clin Dent.2018;29(spec no A):A1-A9.
20. Serrano J, Escribano M, Roldán S, et al. Efficacy of adjunctive anti-plaque chemical agents in managing gingivitis: a systematic review and meta-analysis. J Clin Periodontol. 2015;42 suppl 16:S106-S138.
21. Gunsolley JC. A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J Am Dent Assoc. 2006;137(12):1649-1657.
22. Valkenburg C, Van der Weijden FA, Slot DE. Plaque control and reduction of gingivitis: the evidence for dentifrices. Periodontol 2000. 2019;79(1):221-232.
23. Paraskevas S, van der Weijden GA. A review of the effects of stannous fluoride on gingivitis. J Clin Periodontol. 2006;33(1):1-13.
24. Muhler JC, Radike AW, Nebergall WH, Day HG. The effect of a stannous fluoride-containing dentifrice on caries reduction in children. J Dent Res. 1954;33(5):606-612.
25. Muhler JC, Radike AW, Nebergall WH, Day HG. Effect of a stannous fluoride-containing dentrifice on caries reduction in children. II. Caries experience after one year. J Am Dent Assoc. 1955;50(2):163-166.
26. Nevitt GA, Witter DH, Bowman WD. Topical applications of sodium fluoride and stannous fluoride. Public Health Rep. 1958;73(9):847-850.
27. Makin SA. Stannous fluoride dentifrices. Am J Dent. 2013;26(spec no A):3A- 9A.
28. White DJ. A "return" to stannous fluoride dentifrices. J Clin Dent. 1995;6 spec no:29-36.
29. Myers CP, Pappas I, Makwana E, et al. Solving the problem with stannous fluoride: formulation, stabilization, and antimicrobial action. J Am Dent Assoc. 2019;150(4S):S5-S13.
30. Tinanoff N. Review of the antimicrobial action of stannous fluoride. J Clin Dent. 1990;2(1):22-27.
31. Hines D, Xu S, Stranick M, et al. Effect of a stannous fluoride toothpaste on dentinal hypersensitivity: in vitro and clinical evaluation. J Am Dent Assoc. 2019;150(4S):S47-S59.
32. West NX, He T, Zou Y, et al. Bioavailable gluconate chelated stannous fluoride toothpaste meta-analyses: effects on dentine hypersensitivity and enamel erosion. J Dent. 2021;105:103566.
33. Fiorillo L, Cervino G, Herford AS, et al. Stannous fluoride effects on enamel: a systematic review. Biomimetics (Basel). 2020;5(3):41.
34. Johannsen A, Emilson CG, Johannsen G, et al. Effects of stabilized stannous fluoride dentifrice on dental calculus, dental plaque, gingivitis, halitosis and stain: a systematic review. Heliyon. 2019;5(12):e02850.
35. Hart RK. The thermal oxidation of tin. Proc Phys Soc B.1952;65(12):955.
36. Hunter ML, West NX, Hughes JA, et al. Relative susceptibility of deciduous and permanent dental hard tissues to erosion by a low pH fruit drink in vitro. J Dent. 2000;28(4):265-270.
37. Lussi A, Megert B, Eggenberger D, Jaeggi T. Impact of different toothpastes on the prevention of erosion. Caries Res. 2008;42(1):62-67.
38. A group of experts. Clinical effectiveness of some fluoride-containing toothpastes. Bull World Health Organ. 1982;60(4):633-638.
39. Pettine M, Millero FJ, Macchi G. Hydrolysis of tin(II) in aqueous solutions. Anal Chem. 1981;53(7):1039-1043.
40. Food Drug Administration. Oral health care drug products for over-the-counter human use; antigingivitis/antiplaque drug products; establishment of a monograph. Proposed Rule. Fed Regist. 2003;68(103):31937-32322.
41. Desmau M, Alsina MA, Gaillard JF. XAS study of Sn speciation in toothpaste. J Anal At Spectrom. 2021;36(2):407-415.
42. Burke MR, Gambogi RJ, Simone AJ, Williams MI. The scientific rationale and development of an optimized stannous fluoride dentifrice, Part 1. Compend Contin Educ Dent. 1997;18 spec no:2-9.
43. Mankodi S, Petrone DM, Battista G, et al. Clinical efficacy of an optimized stannous fluoride dentifrice, Part 2: a 6-month plaque/gingivitis clinical study, northeast USA. Compend Contin Educ Dent. 1997;18 spec no:10-15.
44. Williams C, McBride S, Bolden TE, et al. Clinical efficacy of an optimized stannous fluoride dentifrice, Part 3: a 6-month plaque/gingivitis clinical study, southeast USA. Compend Contin Educ Dent. 1997;18 spec no:16-20.
45. Li Y, Suprono M, Mateo LR, et al. Solving the problem with stannous fluoride: extrinsic stain. J Am Dent Assoc. 2019;150(4S):S38-S46.
46. Duffield JR, Williams DR, Kron I. Speciation studies of the solubility and aqueous solution chemistry of tin(II)- and tin(IV)-pyrophosphate complexes. Polyhedron. 1991;10(3):377-387.
47. Vinant M, Agbo-Godeau S, Plantier F, et al. Oral contact stomatitis related to toothpaste use: a report of 15 cases. JEADV Clin Prac. 2024;3(2):672-675.
48. Center for Drug Evaluation, Research. FDA Adverse Event Reporting System (FAERS) Public Dashboard. US Food and Drug Administration website. December 7, 2023. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard. Accessed September 19, 2024.
49. Seriwatanachai D, Triratana T, Kraivaphan P, et al. Effect of stannous fluoride and zinc phosphate dentifrice on dental plaque and gingivitis: a randomized clinical trial with 6-month follow-up. J Am Dent Assoc. 2019;150(4S):S25-S31.
50. Hu D, Li X, Liu H, et al. Evaluation of a stabilized stannous fluoride dentifrice on dental plaque and gingivitis in a randomized controlled trial with 6-month follow-up. J Am Dent Assoc. 2019;150(4S):S32-S37.
51. Gumber HK, Louyakis AS, Sarma T, et al. Effect of a stannous fluoride dentifrice on biofilm composition, gene expression and biomechanical properties. Microorganisms. 2022;10(9):1691.
52. Fine N, Barbour A, Kaura K, et al. Effects of a stabilized stannous fluoride dentifrice on clinical, immunomodulatory, and microbial outcomes in a human experimental gingivitis model. J Periodontol.2024;95(5):421-431.
53. Zhang S, Govindaraju GV, Cheng CY, et al. Oxidative stability of chelated Sn(II)(aq) at neutral pH: the critical role of NO3− ions. Sci Adv.2024;10(40). doi: 10.1126/sciadv.adq0839.
54. Chakraborty B, Seriwatanachai D, Triratana T, et al. Antibacterial effects of a novel stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP): in vitro study and randomized controlled trial. Compend Contin Educ Dent.2024;45 suppl 3:12-20.
55. Lee S, Li Y, Mateo L, et al. A 6-month randomized controlled trial to measure the efficacy of a stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) on dental plaque and gingivitis. Compend Contin Educ Dent. 2024;45 suppl 3:21-29.
56. Liu Y, Lavender S, Ayad F, et al. Effect of a stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) on dentin hypersensitivity: in vitro study and randomized controlled trial. Compend Contin Educ Dent. 2024;45 suppl 3:30-39.
57. Cabelly A, Bankova M, Darling J, et al. Stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) reduces oral malodor: a randomized clinical study. Compend Contin Educ Dent. 2024;45 suppl 3:40-45.
58. Elias-Boneta AR, Mateo LR, D'Ambrogio R, et al. Efficacy of a novel stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) in extrinsic tooth stain removal: a randomized controlled trial. Compend Contin Educ Dent. 2024;45 suppl 3:46-52.
59. Ramsay DS. Patient compliance with oral hygiene regimens: a behavioural self-regulation analysis with implications for technology. Int Dent J.2000;suppl creating a successful:304-311.
60. Bakdash B. Oral hygiene and compliance as risk factors in periodontitis. J Periodontol. 1994;65(suppl 5S):539-544.