Pearl, a dental artificial intelligence company based in Los Angeles, has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Second Opinion® 3D platform. According to the company, this clearance makes Pearl the first and only dental AI company with FDA-cleared solutions for both two-dimensional and three-dimensional dental radiologic image analysis.
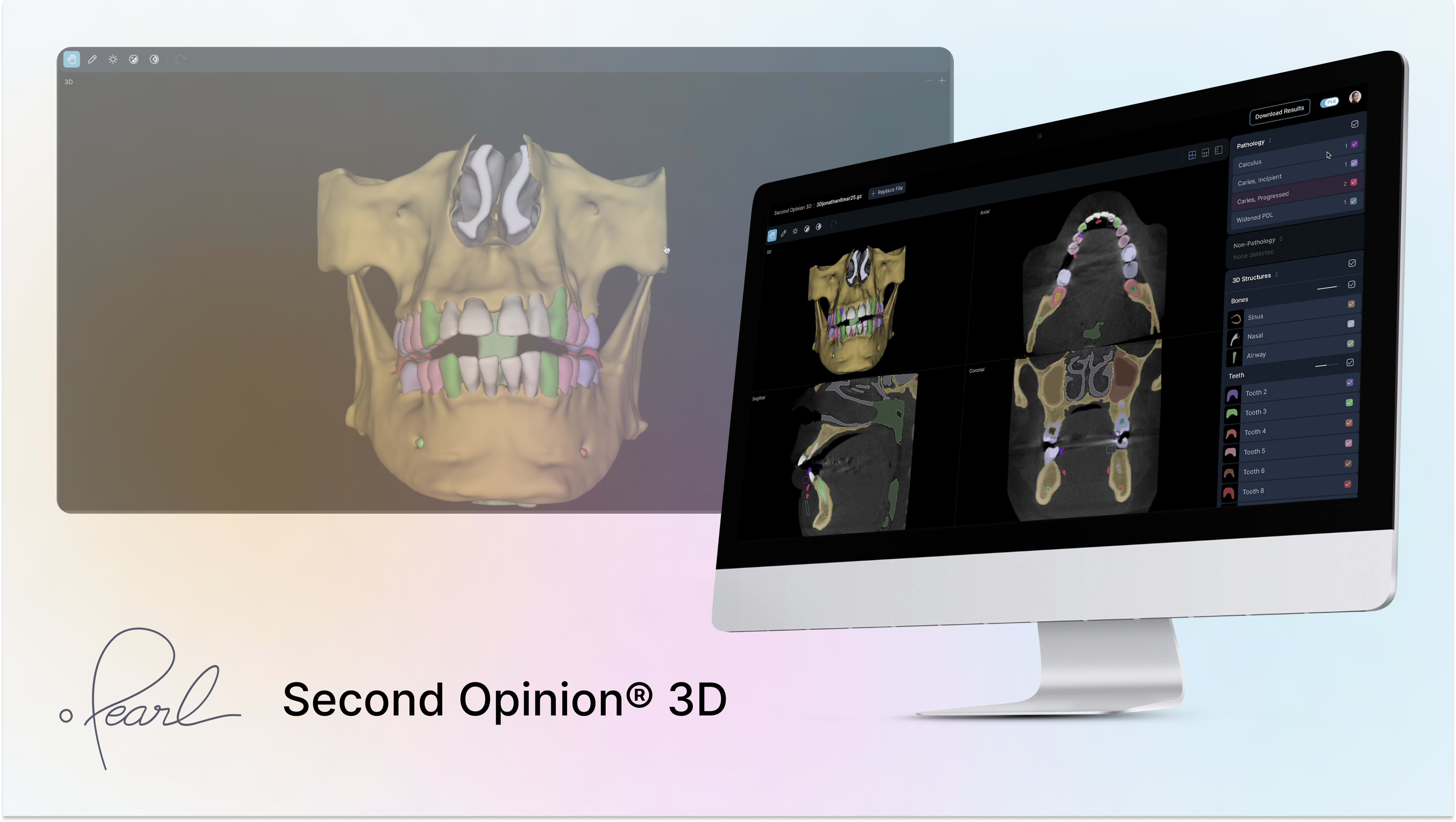
The newly cleared platform extends Pearl’s existing AI capabilities to cone beam computed tomography (CBCT) imaging. According to the company, Second Opinion® 3D enables automated identification of key anatomical structures in 3D scans, including dentition, maxilla, mandible, inferior alveolar canal and mental foramen, maxillary sinus, nasal space, and airway.
“Pearl’s mission has always been to deliver the most advanced and clinically trusted AI solutions in dentistry,” said Ophir Tanz, founder and CEO of Pearl. “Becoming the first company to achieve FDA clearance for both 2D and 3D radiologic analysis isn’t just a milestone for us — it’s a milestone for dentistry.”
According to the company, Second Opinion® 3D is intended to help dental professionals review CBCT scans with increased speed, accuracy, and visual clarity. The system generates AI-supported visualizations designed to support diagnostic and treatment planning workflows across specialties such as implantology, orthodontics, oral surgery, and airway management.
The FDA clearance was granted following bench performance testing. According to Pearl, the software demonstrated high segmentation accuracy for all targeted anatomical structures, with Dice Similarity Coefficient scores exceeding clinical thresholds.
Pearl’s original Second Opinion® platform remains an FDA-cleared AI tool for chairside pathology detection in 2D radiographs. With the addition of 3D functionality, Pearl now offers a platform that, according to the company, is the only one with FDA clearance for use across both major dental imaging modalities.