Abstract: This case report highlights the importance of periodic dental examinations in identifying progressive hypercementosis and its rare complication, root concrescence, which can complicate future dental extractions. Hypercementosis may be idiopathic or secondary to either local factors or systemic disorders. However, evolution to concrescence raises questions about future treatment approaches should the involved teeth become nonrestorable and require extraction. This article presents a case of generalized hypercementosis affecting multiple quadrants with progression. The severe hypercementosis led to concrescence of Nos. 25/26 and 29/30 surrounded by one joint lamina dura. Generalized wear was noted on all occlusal and incisal surfaces of dentition with periodontal pocket depths of teeth Nos. 29 and 30 buccally of 3 mm and 2 mm and distally of 3 mm and 4 mm, respectively. Periapical radiographs from full-mouth series x-rays revealed that the roots of both teeth were grossly thickened, bulbous, and blunted. Because of a lack of contributory medical history, the presumptive diagnosis was hypercementosis sequela to occlusal trauma.
Hypercementosis was defined by Gardner and Goldstein in 1931 as “an excessive growth of cementum of the tooth,” observed radiographically as circumscribed cementum hyperplasia.1 This condition is characterized by an excessive, non-neoplastic deposition of radicular cementum and may involve a single tooth, several teeth, or most of the dentition (ie, generalized).2 In many instances, hypercementosis affects the premolar teeth and presents in bilateral symmetric distribution. Radiographically, hypercementosis is characterized by an overgrowth of cementum contiguous with normal radicular cementum and is contained within the boundaries of the periodontal ligament (PDL) and lamina dura.2,3
Hypercementosis may present secondary to either local factors or systemic disorders; however, in most cases it represents an idiopathic, age-related phenomenon. Systemic disturbances associated with more generalized hypercementosis include Paget’s disease of bone, thyroid goiter, rheumatic fever, arthritis, acromegaly, and vitamin A deficiency; hereditary factors may also be implicated, especially in younger patients.4 Instances of local origin usually involve one tooth or a small group of teeth and include periapical pathosis, parafunctional occlusal trauma, and lack of functional opposition as seen in hypercementosis of impacted teeth.2,3 Periodontitis as an etiologic factor in the formation of hypercementosis has been undocumented in the literature.5
The purpose of this article is to report a case of generalized hypercementosis with concomitant and acquired concrescence, affecting the right mandibular second premolar and first molar in a 62-year-old female patient with discussion of possible treatment modifications and the importance of periodic examinations.
Case Report
This study received exemption from the Institutional Review Board of Midwestern University College of Dental Medicine-Arizona. A 62-year-old female patient presented for her 6-month recare cleaning, examination, and full-mouth series x-rays (FMX) in 2025. Oral cancer screening was completed for extra- and intraoral lesions with results within normal limits. Clinical examination revealed a full complement of dentition with the exception of teeth Nos. 3, 19, and all third molars. Tooth No. 19 had an endosseous implant that was fully restored. Right mandibular dentition were free of caries with resin restorations on teeth Nos. 29 and 31 and a nonsurgical root canal with a porcelain-fused-to-metal crown on tooth No. 30.
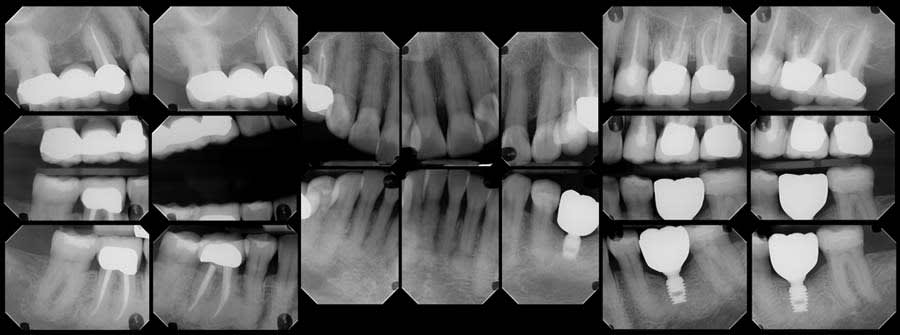
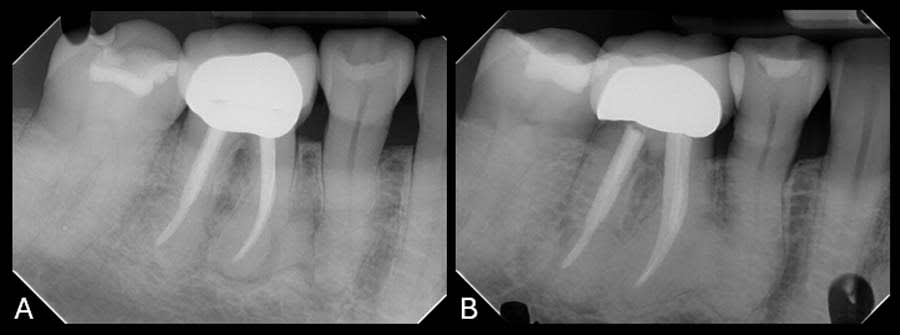
Upon review of the FMX, multiple quadrants of hypercementosis were noted, including all mandibular quadrants and the maxillary left quadrant, and an electric pulp test of the mandibular right posterior quadrant demonstrated vital pulps, with the exception of tooth No. 30 (Figure 1). Teeth Nos. 29 and 30 had no mobility and were percussion negative with periodontal pocket depths buccally of 3 mm and 2 mm and distally of 3 mm and 4 mm, respectively. There was no pain on percussion of tooth No. 31. From the periapical radiographs, the roots of teeth Nos. 29 and 30 were noted to be grossly thickened, bulbous, and blunted due to a fairly symmetrical deposition of a radiopaque material on their surfaces (Figure 2, right). Also noted, both the second premolar and first molar had obvious concrescence of cementum, most pronounced at the first molar (Figure 2, right). In addition, tooth No. 30 incipient PDL space widening and generalized posterior alveolar osseous bone loss were also noted.
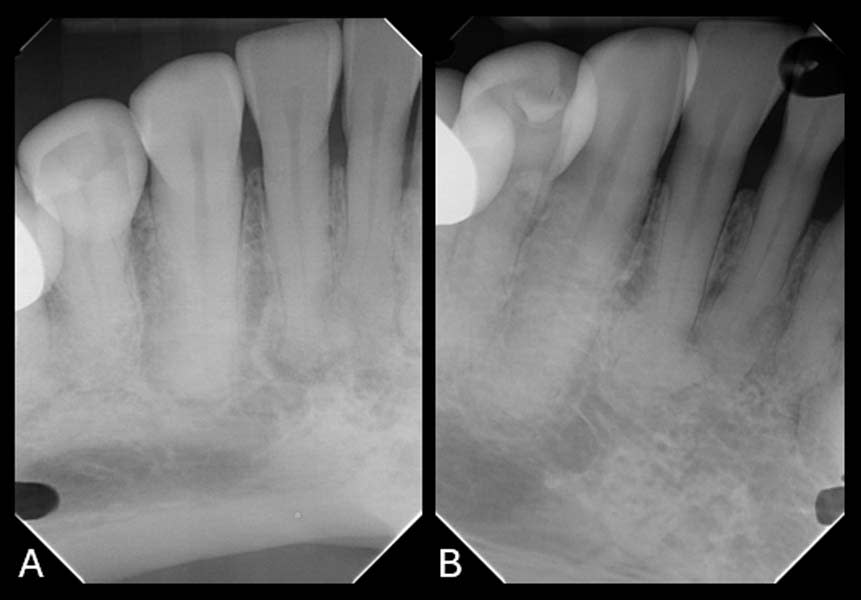
To establish a differential diagnosis, in addition to testing both teeth Nos. 29 and 30 for endodontic/periodontal disease, the clinician reviewed the patient’s systemic health and referenced a previous FMX captured in 2018, which demonstrated a progression of cementum deposition (Figure 2, left). Cementum deposition progression to concrescence of Nos. 25/26 could also be noted (Figure 3).
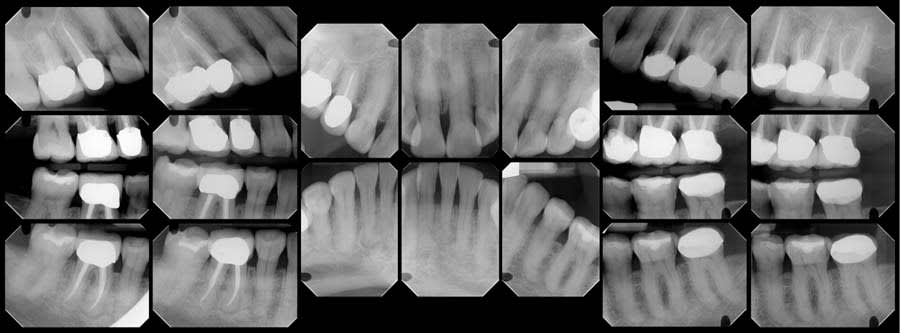
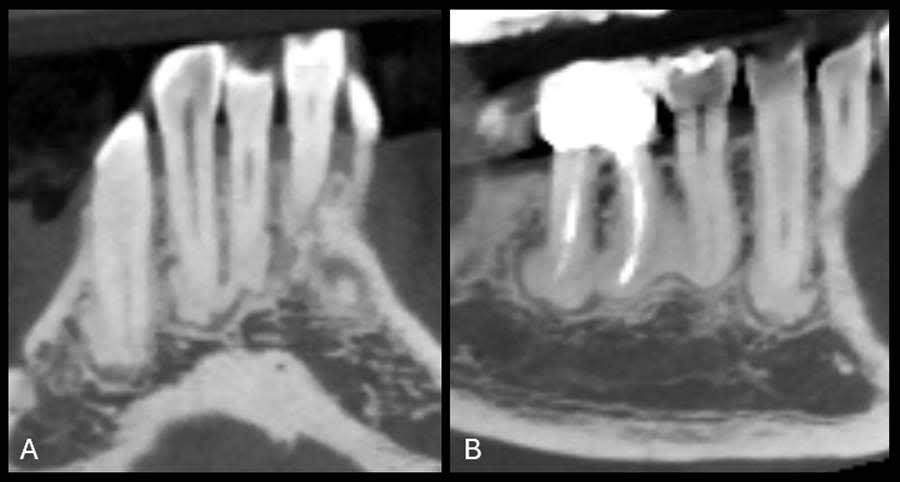
A review of the patient’s dental history revealed that she was never diagnosed with periodontitis, and bone loss had been attributed to physiological aging, gingival recession, and increased biomechanical loading. Tooth No. 19 had been extracted due to secondary caries and not periodontal conditions. The 2018 FMX exhibited generalized hypercementosis, including the mandibular right, mandibular central, and maxillary left posterior areas, and no evidence of concrescence (Figure 4). In 2022, the patient had a cone-beam computed tomography (CBCT) scan in preparation of the No. 19 implant where hypercementosis with concrescence could be seen with Nos. 25/26 and 29/30 (Figure 5).
This clinical and radiographic presentation prompted the exclusion of alternative diagnoses. The patient’s medical history was negative for any systemic condition leading to generalized hypercementosis such as Paget’s disease or hyperpituitarism. The clinical and radiological signs were consistent with severe generalized hypercementosis with localized areas of concrescence due to heavy parafunctional habits and increased biomechanical loading.
Discussion
Cementum is a bone-like mineralized tissue secreted by cementoblasts on the surface of root dentin and plays a critical role in anchoring the PDL to the root.6 Throughout the functional life of a tooth, cementum continues to form incrementally in response to functional demands. When this appositional activity becomes excessive, beyond the physiologic limits of the tooth, hypercementosis, also referred to as cementum hyperplasia, results.7 Radiographically excessive abnormal thickening of cementum along the root surface presents as an enlarged, rounded root surface on a portion, or all, of the root surface.3
Hypercementosis is divided into two types, either as diffuse or focal; either type may affect a single tooth or be generalized across the dentition. Diffuse hypercementosis involves broad coverage of the root surface, whereas focal forms are limited to discrete regions.8 These morphological variations suggest that hypercementosis may have multiple etiologies, both physiological and pathological. The etiology of hypercementosis has been described as idiopathic or in association with a variety of local or systemic conditions.9 Known associated systemic disorders include Paget’s disease,10 Gardner syndrome,11 acromegaly,1,10 hyperthyroidism,12 and immune disorders evidenced as rheumatic fever and arthritis.13 Evidence of gender predilection has been presented in the literature, documenting females having 10 times the incidence of hypercementosis than men.9 Additionally, sources have indicated there is an increased incidence with age and also increased frequent involvement of mandibular first molars, followed by mandibular and maxillary second premolars.14
The excessive hypercementosis involving the mandibular right second premolar and first molar noted in this report suggests a possible adaptive or compensatory response to increased occlusal forces.15 Hypercementosis increases the total root surface area, which may enhance tooth stability under excessive load. In this case, the marked cementum deposition, particularly in the apical halves of roots, may reflect a biological adaptation to functional demands such as occlusal trauma. The cementum apposition could provide reinforcement and support in areas of periodontal tissue loss or excessive loading.
The maintenance of the peripheral PDL and lamina dura in this case indicates that the condition was likely non-inflammatory in origin and unrelated to systemic disease. CBCT imaging confirmed the presence of a cementum bridge between the affected teeth, consistent with concrescence.
Concrescence is a rare developmental anomaly characterized by the fusion of adjacent teeth through cementum alone. Because the fusion is limited to cementum, clinical detection is often difficult, and conventional 2-dimensional radiographic imaging may misinterpret it as simple root proximity or overlap. The etiology of concrescence remains poorly understood. It may arise during tooth development or post-eruption, with contributing factors including local trauma, crowding, loss of interdental bone, chronic inflammation, or lack of opposing dentition.5 It has been proposed that acquired concrescence may develop as a consequence of, or be exacerbated by, hypercementosis, with both conditions sharing a common pathophysiological basis involving unchecked cementogenesis.16
Additionally, the patient reported two separate phases of orthodontic treatment. Phase one orthodontic treatment took place at age 10 with bilateral extraction of premolars. A second phase of treatment occurred at age 20 and was limited to the maxillary arch with extraction of premolars. This history is particularly relevant, as the interaction between orthodontic forces and teeth with hypercementosis remains poorly understood. Current literature offers limited data on how hypercementosis results from or affects tooth movement. The patient’s long-term occlusal history, including early extractions and altered functional loading, may have contributed to the development of both hypercementosis and concrescence.
The etiology of generalized forms remains elusive, and co-occurrence with concrescence in this patient raises further questions. Specifically, it is unclear why some teeth with hypercementosis progress to concrescence while others remain as singular roots with hypercementosis. This observation suggests a multifactorial process possibly involving occlusal trauma, parafunctional habits, lack of functional loading from unopposed teeth, or the presence of low-grade, chronic inflammation.
In the present case, sequential radiographs demonstrated significant progression in cementum deposition over time. The persistence of normal periodontal structures, combined with the pattern of cementum growth, supports the hypothesis that occlusal load may contribute to the progression of hypercementosis to concrescence. The patient denied systemic disease, other than hypothyroidism.
The patient’s atypical distribution raises important questions about cementum biology, particularly regarding the deposition of cementum in regions subject to differing functional loads. Typically, posterior teeth are exposed to high occlusal forces, which may influence cementum proliferation. Reviewing intraoral photographs captured in 2025, heavier than expected anterior occlusion was noted. However, the presence of concrescence in the mandibular anterior region, where occlusal forces should be minimal, suggested that occlusal forces may have been involved and may have played a role in the pathogenesis of cementum hyperplasia.
From a clinical standpoint, concrescence often goes undetected until complications arise during procedures such as extractions, endodontic treatment, or orthodontic movement. Failure to identify this condition preoperatively can result in unforeseen surgical challenges and potentially adverse outcomes. Moreover, undiagnosed concrescence may carry medicolegal implications, particularly if complications occur. The present case underscores the need for increased awareness of concrescence, especially in patients with atypical root morphology or potential orthodontic treatment. Use of appropriate imaging tools is essential to ensure accurate diagnosis and effective treatment planning.
Conclusion
The concomitant presence of generalized hypercementosis and concrescence in this patient underscores the potential role of excessive occlusal forces in cementum overgrowth. Although the condition appears to involve various local and compensatory mechanisms, the precise contribution of individual factors—such as orthodontic movement or parafunction—remains unclear. Further studies are needed to elucidate the complex etiology of cementum hyperplasia and its progression to concrescence.
About the Authors
Olysia Takla, DMD
Clinical Assistant Professor, Midwestern University College of Dental Medicine, Glendale, Arizona
Ashley L. Madern, DMD, MS
Oral and Maxillofacial Radiologist and Clinical Assistant Professor, Midwestern University College of Dental Medicine, Glendale, Arizona
Karen Berrigan, DMD, MS
Orthodontist and Clinical Assistant Professor, Midwestern University College of Dental Medicine, Glendale, Arizona
Pili Peters, DMD
General Dentist, Durango, Colorado
References
1. Gardner B, Goldstein H. The significance of hypercementosis. Dent Cosmos. 1931:1065-1069.
2. Rairam S, Allurkar S, Reddy SP, et al. A distinctive case report of a coalesced hypercementosed mandibular first molar. IOSR J Dent Med Sci. 2015;14(2):71-73.
3. Asykarie INA, Ramadhan FR, Firman RN. Multiple hypercementosis–a case report of an incidental finding on panoramic radiograph. J Radiol Dentomaxillofac Indones. 2022;6(1):17-20.
4 Leider AS, Garbarino VE. Generalized hypercementosis. Oral Surg Oral Med Oral Pathol. 1987;63(3):375-380.
5. Romito LM. Concrescence: report of a rare case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(3):325-327.
6. Diekwisch TG. The developmental biology of cementum. Int J Dev Biol. 2001;45(5-6):695-706.
7. Massé L, Garot E, Maureille B, Le Cabec A. Insights into the aetiologies of hypercementosis: a systematic review and a scoring system. Arch Oral Biol. 2023;146:105599.
8. Barrett AW. Exuberant hypercementosis. Br Dent J. 2013;215(12):602.
9. Bürklein S, Jansen S, Schäfer E. Occurrence of hypercementosis in a German population. J Endod. 2012;38(12):1610-1612.
10. Elsayed S, Alolayan A, Farghal L, Ayed Y. Generalised hypercementosis in a young female seeking extraction: revision and update of surgical technique. J Coll Physicians Surg Pak. 2019;29(11):1111-1113.
11. Arendt DM, Frost R, Whitt JC, Palomboro J. Multiple radiopaque masses in the jaws. J Am Dent Assoc. 1989;118(3):349-351.
12. Kupfer I. Correlation of hypercementosis with toxic goiter; a preliminary report. J Dent Res. 1951;30(5):734-736.
13. Chavan PS, Rairam SG, Patil VS, Ratnakar P. Hypercementosis: an overview. RCDS J. 2021;1:14-17.
14. Mupparapu M, Shi KJ, Ko E. Differential diagnosis of periapical radiopacities and radiolucencies. Dent Clin North Am. 2020;64(1):163-189.
15. Commuzie AG, Steele DG. Enlarged occlusal surfaces on first molars due to severe hypercementosis: examples from prehistoric coastal populations of Texas. Am J Phys Anthropol. 1989;78(1):9-15.
16. Dargue A. ‘Acquired’ concrescence causing surgical complications – a report of two cases. Oral Surg. 2017;10(4):e92-e97.