Farah Mirza, BDS, MS; Melissa Nelson, MS; Janice Ambers, BA; Kimberly Milleman, RDH, MS, PhD; Jeffery Milleman, DDS, MPA; and Marilyn Ward, DDS
Abstract: The objective of this study was to evaluate the effect of different interdental oral cleaning modalities on gingivitis and plaque following a 6-week period of home use. This was a randomized, four-arm, parallel design clinical trial. Study subjects were manual toothbrush (MTB) users with moderate to severe gingivitis, aged 18 to 65 years. Subjects were required at baseline to have a gingival bleeding score of ≥1 on at least 50 gingival sites per the Gingival Bleeding Index (GBI) and to have an overall plaque score of ≥0.6 per the Rustogi Modified Navy Plaque Index (RMNPI) following a 3- to 6-hour plaque accumulation period. Subjects were randomly assigned to use one of four oral care cleaning modalities: (1) NON group: MTB alone, (2) FLS group: MTB plus string floss, (3) IDB group: MTB plus an interdental brush, or (4) CPF group: MTB plus the Philips® Sonicare® Cordless Power Flosser with the Quad Stream nozzle. Efficacy measures (Modified Gingival Index [MGI], GBI, and RMNPI) and safety were assessed at baseline, 2 weeks, and 6 weeks. The primary efficacy endpoint was the reduction in gingival inflammation from baseline to week 6 as measured by the MGI. Of the 372 subjects randomized in the study, 364 completed a post-baseline MGI evaluation and were included in the analyses. The adjusted mean percent reduction in gingival inflammation from baseline to week 6 was -2.10% for the NON group, 2.82% for the FLS group, 2.60% for the IDB group, and 29.10% for the CPF group. Pairwise comparisons indicated that the CPF group was statistically significantly different from the NON, FLS, and IDB groups (P< .0001). In conclusion, adjunctive use of the Philips Sonicare Cordless Power Flosser with the Quad Stream nozzle and an MTB showed statistically better results in term of reducing gingival inflammation following 6 weeks of home use when compared to an MTB alone, an MTB used with string floss, and an MTB used with an interdental brush.
Good oral hygiene practices that involve the regular removal of food debris and dental plaque biofilm from the surfaces of teeth are important in maintaining a healthy and functional dentition. It has been shown that oral health has a direct impact on people's daily life.1 Functional and healthy dentition increases oral health quality of life, whereas painful or functional complaints are associated with impaired quality of life.2
As a common means to clean teeth, the bristles of a typical toothbrush reach most facial and lingual surfaces, however they have limited access to deeper interdental surfaces. Adjunctive measures such as string floss and interdental brushes are often recommended for interdental cleaning. It has been shown that combining flossing and toothbrushing is more successful in reducing gingivitis and plaque than toothbrushing alone.3 However, adequate flossing remains a challenge for many people because of discomfort, difficulty of use, or dexterity issues. As an alternative to floss and interdental brushes, powered flossers utilizing jets of water are often recommended as an adjunct to standard toothbrushing. Power flossers with water jets have been shown to decrease plaque and interproximal bleeding,4 benefits that, along with ease of use, may lead to higher patient compliance with dental professional recommendations.
Toothbrushing and interdental cleaning remain the primary means to prevent periodontal diseases. Currently, dental professionals tailor oral hygiene instructions to individual patients because general recommendations concerning the ideal oral hygiene devices to use and procedures to follow remain inconclusive.5 Additional studies are needed to investigate the oral health benefits of different interdental cleaning modalities. This study helps fill that gap by evaluating changes in gingival health and plaque level following manual toothbrushing combined with various interdental cleaning modalities, including string floss, an interdental brush, and the Philips® Sonicare® Cordless Power Flosser with the Quad Stream nozzle.
Materials and Methods
This was a prospectively planned, randomized, parallel, single-blinded study that was reviewed and approved by an accredited Institutional Review Board (Advarra IRB Pro00055532; IRB00000971; Columbia, Maryland). The study's primary objectives were to evaluate the effect of different oral cleaning modalities on gingivitis, as evaluated by the Modified Gingival Index (MGI),6 following a period of 6 weeks of home use, and to simultaneously evaluate the safety of all products. Secondary objectives included an additional gingivitis comparison at a 2-week timepoint and comparisons between the oral cleaning modalities on plaque removal and gingival bleeding at both 2-week and 6-week timepoints. Power analysis for this protocol was based on the primary endpoint of reduction in MGI from baseline to week 6, comparing different cleaning modalities. A sample size of 89 subjects per treatment group was estimated to provide 90% power to detect a difference in the reduction of MGI as small as 0.25 using a two-sided t-test after a Bonferonni adjustment for multiple comparisons.
The three efficacy endpoint measures in this study were the MGI, Gingival Bleeding Index (GBI),7 and Rustogi Modified Navy Plaque Index (RMNPI).8 The MGI and GBI metrics were performed on all evaluable teeth at six sites per tooth, and the RMNPI was recorded at 18 sites per evaluable tooth. The safety endpoints were the adverse changes in the oral cavity upon oral examination and/or subject interview that were "possibly related" or "related" to the test products, adverse events (AEs) that occurred in/around the oral cavity, and any events that were unanticipated adverse device effects.
Subject recruitment included generally healthy individuals that were aged 18 to 65 years, nonsmokers, and regular manual toothbrush users. Subjects were required to have a minimum of 20 scorable teeth (excluding third molars), a minimum average overall RMNPI score of ≥0.6 following a 3- to 6-hour plaque accumulation period, and a gingival bleeding score of ≥1 on at least 50 gingival sites per the GBI. Subjects were excluded if they were regular power toothbrush users or regular users of dental floss or other interdental cleaning products.
Study participants were randomized to one of four treatment groups according to a predefined randomization schedule and stratified by gender. All subjects used a manual toothbrush (MTB) with or without an additional cleaning modality. The four treatment groups were: (1) NON group: MTB alone with no interdental cleaning modality, (2) FLS group: MTB plus string floss (unflavored waxed), (3) IDB group: MTB plus an interdental brush, or (4) CPF group: MTB plus the Philips Sonicare Cordless Power Flosser used in the clean mode with high intensity along with the Quad Stream nozzle. All subjects were provided with standard fluoride-containing dentifrice to use for the duration of the study and were prohibited from using any other at-home oral hygiene treatments. Subjects were instructed to brush at home with the MTB for 1 minute twice daily. For the FLS, IDB, and CPF groups, subjects were instructed to use the interdental cleaning modality once daily in the evening per provided instructions. At each study visit, subjects were required to present with 3 to 6 hours of plaque accumulation. Subjects were provided with a compliance diary to record each interdental cleaning and brushing encounter.
Randomization and subject instruction on treatment regimen use were performed by unblinded study personnel who did not perform any evaluations or assessments related to study endpoints. Efficacy outcomes included the observed response, the reduction from baseline, and the percent reduction from baseline. An analysis of covariance (ANCOVA) model, with baseline score as the covariate, was used to assess treatment response at each visit and compare the response of the CPF group to the NON, FLS, and IDB groups. All statistical tests were two-sided. As an additional means to assess gingival health changes, each subject's overall gingivitis status was classified as "healthy" (<10% bleeding sites) or "not healthy" (≥10% bleeding sites) per the American Academy of Periodontology (AAP) and European Federation of Periodontology (EFP) criteria at each visit. The number and percentage of subjects transitioning from "not healthy" at baseline to "healthy" at week 2 and week 6 were calculated. Treatment groups were compared using a chi-square test.
Results
Of the 376 subjects screened for this study, four failed screening and 372 were enrolled and randomized to one of the four treatment groups. There were no statistical differences in the distribution of age and gender of subjects between groups (P = .6458 and P = .9977, respectively). Fifteen subjects terminated the study early (13 withdrew, two lost to follow-up).
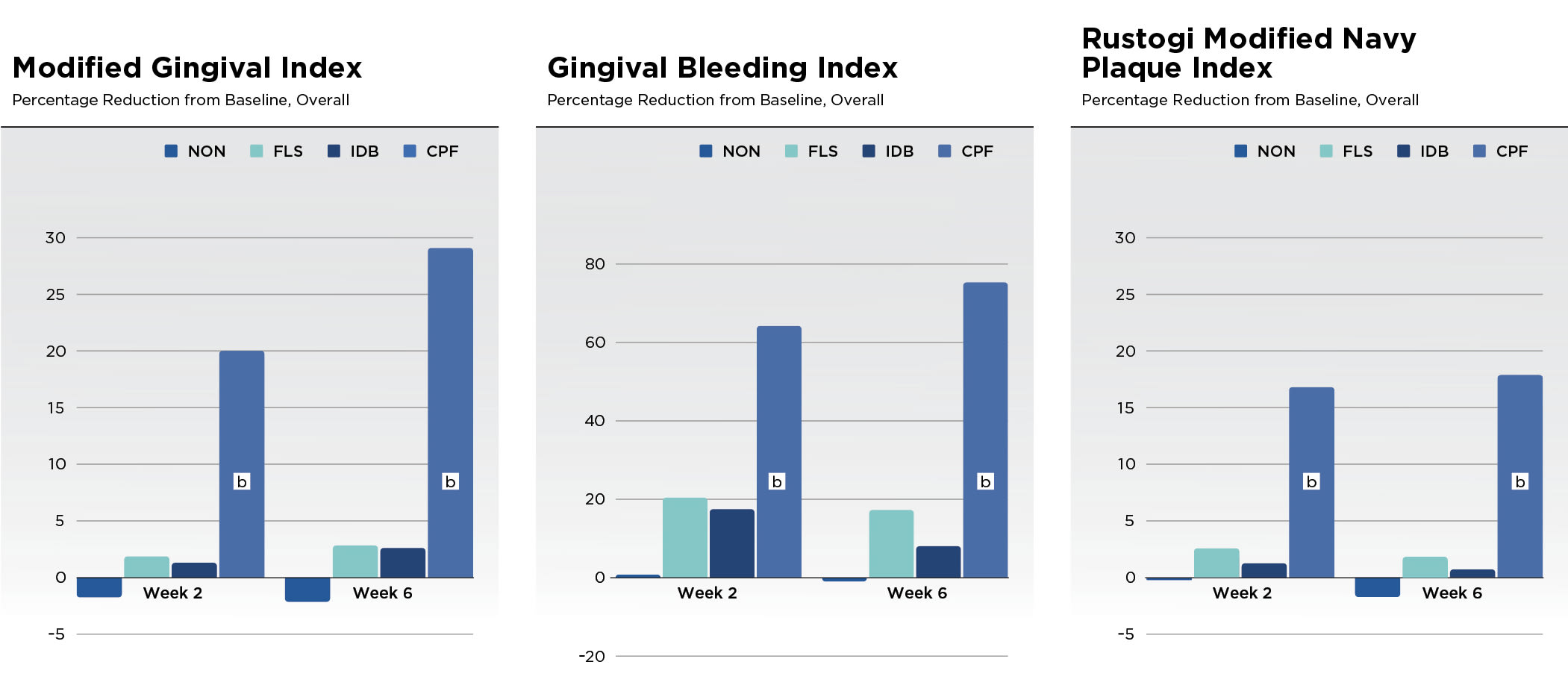
Results for the efficacy scores for all treatments at baseline, week 2, and week 6 can be found in Table 1 for the MGI, GBI, and RMNPI for all subjects completing a post-baseline MGI evaluation. There were no significant differences between treatment groups in the scores at baseline (P > .05) for any of these indices. In addition to Table 1, the adjusted mean percent reduction from baseline in the three indices scores are presented graphically (Figure 1).
Statistical analysis of the data indicated a significant between-group treatment effect (P < .0001) for all indices and timepoints. Pairwise comparisons showed statistically better results for the CPF group compared to the NON, FLS, and IDB groups (P < .0001) in terms of reduction in MGI, GBI, and RMNPI at both week 2 and week 6. At week 6, the improvement in MGI for CPF was at least nine times higher than the comparator groups. The reduction in MGI was 29.10% for CPF versus -2.10% for NON, 2.82% for FLS, and 2.60% for IDB groups. At week 6, the improvement in GBI for CPF was at least four times higher than the comparator groups. The reduction in GBI was 75.30% for CPF versus -0.95% for NON, 17.25% for FLS, and 8.03% for IDB groups. At week 6, the improvement in RMNPI for CPF was at least nine times higher than the comparator groups. The reduction in RMNPI was 18.80% for CPF versus -1.23% for NON, 1.83% for FLS, and 0.50% for IDB groups.
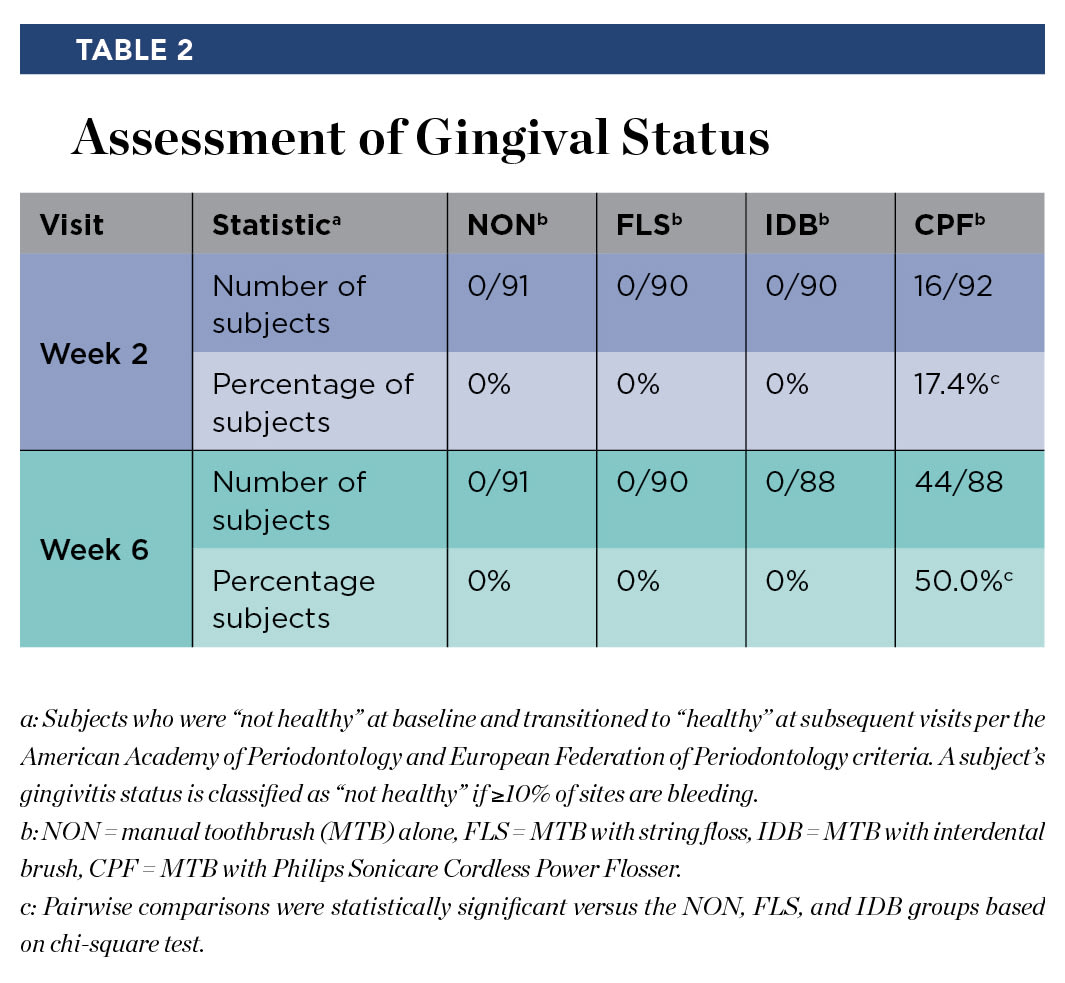
All subjects had ≥10% of gingival sites bleeding at baseline, hence they were considered "not healthy" per the AAP/EFP criteria. Table 2 presents the number and percentage of subjects transitioning from "not healthy" at baseline to "healthy" at week 2 and week 6. Within the CPF group, 16 of 92 subjects (17.4%) transitioned to "healthy" at week 2 and 44 of 88 subjects (50.0%) transitioned to the "healthy" category at week 6. No subjects in the NON, FLS, or IDB groups transitioned to "healthy" at week 2 or week 6. Pairwise comparisons of the treatment difference between the CPF group and the NON, FLS, and IDB groups were statistically significant (P < .0001).
Three AEs were reported in the study: one in each of the NON, FLS, and CPF groups. The AEs were mild in severity. There were no AEs that the investigator classified as either "possibly related" or "related" to the study or the study products.
Discussion and Conclusions
This study investigated whether a cordless power flosser used once daily after manual toothbrushing could improve oral hygiene in subjects over a 6-week period of home use. The results showed that usage of a Philips Sonicare Cordless Power Flosser in the clean mode and at the high intensity setting along with the Quad Stream nozzle following manual toothbrushing resulted in statistically significant gingival health improvements when compared to manual toothbrushing alone, manual toothbrushing with the use of string floss, and manual toothbrushing with the use of an interdental brush. The data also indicates that all products tested were safe for daily use. Further examination of the data in support of the secondary objectives observed at the 2-week timepoint found that the Philips Sonicare Cordless Power Flosser produced the best results (in comparison to the other modalities) in terms of improvements to gingival health outcomes. Examining the remaining secondary objectives indicated that the power flosser also produced the best results in terms of reducing gingival bleeding and plaque at both the 2-week and 6-week timepoints.
The ability to make informed decisions regarding oral care products is important to both dental professionals and consumers. Human clinical studies to determine efficacy and safety of oral care products provide evidence by which these decisions are informed. Examining the gingival status outcome (Table 2) over the course of this study indicates that use of the cordless power flosser resulted in statistically significantly more subjects transitioning from "not healthy" to "healthy." This shift in bleeding from diseased to healthy is a significant benefit to consumers and indicates that the cordless power flosser used in a daily oral care regimen provides an important tool for users to manage oral health.
In conclusion, the Philips Sonicare Cordless Power Flosser with the Quad Stream nozzle used adjunctively with manual toothbrushing was shown to provide statistically significant benefits in the reduction of gingival inflammation, gingival bleeding, and plaque compared to a manual toothbrush alone, a manual toothbrush plus string floss, or a manual toothbrush plus an interdental brush. All products used in the study were safe for home use.
Disclosure
This study was sponsored by Philips Oral Healthcare.
About the Authors
Farah Mirza, BDS, MS
Senior Clinical Development Scientist and Medical-Dental Safety Officer, Philips Oral Healthcare, Bothell, Washington
Melissa Nelson, MS
Associate Director, Biostatistics, Philips Oral Healthcare, Bothell, Washington
Janice Ambers, BA
Manager, Clinical Operations, Philips Oral Healthcare, Bothell, Washington
Kimberly Milleman, RDH, MS, PhD
Director, Compliance Specialist, and Gold Standard Examiner, Salus Research, Inc., Fort Wayne, Indiana
Jeffery Milleman, DDS, MPA
Director, Clinical Operations and Principal Investigator, Salus Research, Inc., Fort Wayne, Indiana
Marilyn Ward, DDS
Clinical Development Director, Philips Oral Healthcare, Bothell, Washington
References
1. Karasneh J, Al-Omiri MK, Al-Hamad KQ, Al Quran FA. Relationship between patients' oral health-related quality of life, satisfaction with dentition, and personality profiles. J Contemp Dent Pract. 2009;10(6):
E049-E056.
2. van de Rijt LJM, Stoop CC, Weijenberg RAF, et al. The influence of oral health factors on the quality of life in older people: a systematic review. Gerontologist. 2020;60(5):e378-e394.
3. Gallie A. Home use of interdental cleaning devices and toothbrushing and their role in disease prevention. Evid Based Dent. 2019;20
(4):103-104.
4. Edlund P, Bertl K, Pandis N, Stavropoulos A. Efficacy of power-driven interdental cleaning tools: a systematic review and meta-analysis. Clin Exp Dent Res. 2023;9(1):3-16.
5. Sälzer S, Graetz C, Dörfer CE, et al. Contemporary practices for mechanical oral hygiene to prevent periodontal disease. Periodontol 2000. 2020;84(1):35-44.
6. Lobene RR, Weatherford T, Ross NM, et al. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8(1):3-6.
7. Van der Weijden GA, Timmerman MF, Nijboer A, et al. Comparison of different approaches to assess bleeding on probing as indicators of gingivitis. J Clin Periodontol. 1994;21(9):589-594.
8. Rustogi KN, Curtis JP, Volpe AR, et al. Refinement of the Modified Navy Plaque Index to increase plaque scoring efficiency in gumline and interproximal tooth areas. J Clin Dent. 1992;3(suppl C):C9-C12.