Aileen Cabelly, MS; Mania Bankova, PhD; Jason Darling, BS; Tracy Bariexca, BS; Catalina Monroy; Deyu Hu, DDS, MS; Luis R. Mateo, MA; Pamela Monty, BS, RDH; Robert D’Ambrogio, BS; Maria Ryan, DDS, PhD; and Yun-Po Zhang, PhD, DDS (Hon)
Abstract: Background: Oral malodor, whether from systemic disease, dietary sources, or bacteria in the oral cavity, can negatively impact patients' quality of life. Oral malodor due to bacteria in the oral cavity can be managed by mechanically or chemically removing bacteria. Dentifrices are ideal vehicles to deliver therapeutic active ingredients that promote and maintain oral health since most consumers brush their teeth daily. Consumer preference drives consistency in oral hygiene routine. This study first identified a favorite flavor via consumer flavor testing and then measured the clinical efficacy of the dentifrice with a new flavor formulation to reduce malodor. Methods: Consumer testing was conducted via an online product evaluation questionnaire to gauge consumer flavor preferences for stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP). In a 3-week randomized, single-center, double-blind clinical study, the malodor reduction ability of SNaP was compared to the negative control toothpaste containing 0.76% sodium monofluorophosphate via the organoleptic method. Results: Consumer testing was used to determine a winning flavor for the new flavor formulation of SNaP tested in the clinical study. In this study, after 3 weeks of product use, on average, malodor clinical trial subjects (n = 97) randomized into the SNaP group had a 32.7% malodor score reduction from baseline (P < .001) 12 hours post-brushing compared to a 9.4% reduction in the negative control group. Relative to the negative control group, the SNaP group had a statistically significant reduction of 25.7% (P < .001) in oral malodor via organoleptic scores. Conclusions: SNaP toothpaste delivered superior malodor reduction 12 hours post-brushing when compared to a negative control toothpaste. Practical Implications: Incorporating therapeutic active ingredients like stannous fluoride into toothpaste is an effective way to deliver oral health benefits, such as caries prevention, reduction in gingivitis and dentin hypersensitivity, and protection against enamel erosion and bad breath.
Oral malodor, also known as halitosis, is a common and manageable condition.1,2 Prevalence measures vary by population; however, a recent systematic review of oral malodor literature estimated that one in four people have some measure of malodor.2 Oral malodor is detrimental to patients, as those experiencing it can report a reduction in social function and emotional well-being.3 Oral malodor is significantly associated with social anxiety.4 Further, the same bacteria responsible for oral malodor can also cause periodontal disease.5
Oral malodor has multiple sources.2 Systemic issues, such as liver failure, can impact oral malodor, as can dietary choices, smoking, and alcohol consumption.2 Oral malodor can also be caused by bacteria present in plaque, tongue coating, trapped food, and other substances in the mouth.5,6Oral hygiene routines that remove bacteria chemically and mechanically can improve malodor.7 Dentifrices address oral malodor through multiple routes. First, flavor-formulated dentifrices deliver a pleasant smell to temporarily freshen breath. Also, antimicrobial formulations of dentifrice address the bacterial-related causes of oral malodor.
Dentifrices are ideal vehicles to deliver therapeutic active ingredients that promote and maintain oral health because most consumers brush their teeth daily. Dentifrices have been formulated to have antimicrobial effects that can disrupt the buildup of bacteria leading to malodor.1 Using stannous fluoride toothpaste when brushing delivers various oral care benefits, including prevention of caries, enamel erosion, and bad breath, reduction of gingivitis and tartar buildup, and decreased dentin hypersensitivity. Stannous fluoride has been found effective in its ability to fight bacteria, including those that produce malodorous compounds.8-10
Along with efficacy, taste is another important attribute of oral care products because it is directly linked to consumer preference and adherence to recommended oral healthcare practices. Formulations with pleasing and delightful taste drive consumer exposure to new oral care products and help ensure consumer adherence to oral hygiene regimens compliance.11,12To deliver whole-mouth health, a toothpaste formulation needs to contain multiple ingredients that work in synergy and complement each other. These ingredients can impact hedonics and mouth sensation and consequently consumer usage behavior, which can translate into enhanced therapeutic value and efficacy for the consumers.
A novel dentifrice containing 0.454% stannous fluoride stabilized with nitrate and phosphates (SNaP) has been developed to provide a wide spectrum of benefits for the oral cavity.13 Its efficacy has been clinically proven to deliver whole-mouth health.10,14-16However, stannous metal ions can present taste challenges as they impart a lasting unpleasant mouth sensation. Stannous fluoride-containing formulations can have an astringent taste that is mostly noticeable after use of the product. Therefore, consumer acceptability of the SNaP toothpaste flavor must be confirmed in addition to clinical efficacy. The aim of this research was to first select a winning flavor for SNaP development and then measure the oral malodor reduction performance of the SNaP toothpaste.
Materials and Methods
Consumer Testing
A new toothpaste formulation, SNaP, was evaluated via consumer testing in the United States and the United Kingdom. Two new flavor profiles of the SNaP toothpaste formula were compared to the formulation of Colgate Total® (Colgate-Palmolive Co., colgatepalmolive.com) available on the market in the United States and the United Kingdom at the time of consumer testing. Eligible consumer testing participants were men and women aged 18 to 70 (inclusive) responsible for buying and/or choosing the oral care products they use, who were also current users of Colgate Total and brushed their teeth at least once daily.
Screening, placement, and product evaluation was conducted online in a representative national sample of Colgate Total product users. Each subject was given one tube of the assigned toothpaste to try in place of their usual product for 14 days. Consumers completed a questionnaire after the 14 days of in-home product use. The questionnaire asked participants to select their level of agreement with different statements regarding products on a five-point scale where "5" meant the statement "describes the toothpaste you tried completely." The flavor rated most favorably by consumer testing participants was used in the formulation of SNaP toothpaste undergoing clinical testing for efficacy to reduce malodor.
Clinical Investigation
In a randomized, single-center, two-cell, double-blind, parallel clinical study, the SNaP toothpaste was tested for its effectiveness in reducing oral malodor.
Products Tested: The two comparator products were the test toothpaste SNaP, which contains 0.454% stannous fluoride stabilized with nitrate and phosphates, and the negative control toothpaste containing 0.76% sodium monofluorophosphate (Colgate-Palmolive Co.). The consumer-preferred flavor was the flavor utilized in the SNaP toothpaste formulation in the malodor clinical study.
Ethics: The study was reviewed and approved by the Institutional Review Board of China Oral Health Foundation located in the International Building at 18-A South Avenue in Zhongguancun, Haidian District, Beijing, 100081. All participating subjects signed an informed consent form.
Study Setting and Location: The study was conducted at the West China Dental Institute of Chengdu in Chengdu, China. The recruitment period was from October 15 to October 18, 2022. The study period was from June 1 to July 2, 2023.
Participant Inclusion and Exclusion: For study inclusion, subjects had to be aged 18 to 70 (inclusive), in good general health, in good oral health based on self-assessment, available for the full duration of the study, with a baseline mean malodor score between 6.0 and 8.0 (inclusive), a minimum of 20 naturally uncrowned teeth (excluding third molars), and no history of allergies to personal care products or other consumer products or their ingredients.
Subjects were excluded from the study if any of the following applied: participation in any other oral clinical studies during the duration of this study; have full or partial dentures; are pregnant or lactating (breastfeeding); use of tobacco products; history of allergies to common oral care ingredients or oral care products; use of phenolic flavored products such as mint flavored candies and chewing gum the morning of the study and during the sampling periods; immunocompromised individuals (eg, HIV, AIDS, immunosuppressive drug therapy); or unable to abstain from eating or drinking due to medical conditions for the post-use treatment evaluation timepoints.
Sample Size: The sample size of 100 subjects (50 per group) was determined based on the overnight organoleptic standard deviation (SD) between products of 2.5, a significance level of α = 0.05, a 10% attrition rate, and an 80% level of power. This study was powered to detect a minimal statistically significant difference between study group means of 4.00.
Randomization and Blinding of Treatment: Subjects were randomized into the SNaP test group or negative control group based on a computer-generated list of random numbers. Each group was assigned to a product in a parallel design. The examiners, subjects, and the study statistician were all blinded to product allocation. Products were overwrapped and coded by the sponsor to preserve blinding.
Intervention: All qualified subjects used a regular fluoride toothpaste containing 0.76% sodium monofluorophosphate for daily hygiene (twice daily brushing, once in the morning and once in the evening, for 2 minutes each time) for 7 days. At the end of the 7-day washout period, all subjects returned to the clinical site and were evaluated for their baseline oral malodor by four trained examiners using a hedonic scale from 1 to 9 (described below). Subjects were instructed to refrain from all oral hygiene (brushing, rinsing, and flossing) and eating and drinking for at least 6 hours prior to each scheduled visit for evaluations. Oral malodor evaluations were conducted at baseline and at the 3-week visit. Baseline evaluations were conducted 12 hours post-brushing with the regular fluoride toothpaste. At baseline, the mean of the scores provided by the four judges constituted a subject's baseline oral malodor score. Subjects then brushed with the assigned toothpastes (twice daily, once in the morning and once in the evening, for 2 minutes on each occasion) for the next 3 weeks. At the 3-week evaluation scheduled visit, all subjects were evaluated again for oral malodor 12 hours post-brushing with the assigned toothpastes.
Scoring Procedure: A specially designed screen was used to hide the identities of the judges and subjects and only permit the judges to be exposed to the breath of each individual. When standing in front of this barrier, with the judges on the opposite side, the subjects were instructed to close their mouth, breathe through their nose, and not swallow for 2 minutes. Subjects placed their mouth over the one end of an autoclaved breathing cylinder and breathed gently. Each of the four trained and calibrated examiners placed their nose at the opposite side and scored oral malodor using the following nine-point hedonic scale: 1 = most pleasant; 2 = very pleasant; 3 = moderately pleasant; 4 = slightly pleasant; 5 = neither pleasant nor unpleasant; 6 = slightly unpleasant; 7 = moderately unpleasant; 8 = very unpleasant; 9 = most unpleasant.
Statistical Methods: For each subject at each evaluation timepoint, the hedonic breath-odor scores assigned by the four judges were averaged to yield a single subject-wise score. Statistical analyses were performed on these average organoleptic hedonic scores. Comparisons of the treatment groups with respect to gender were performed using a chi-square analysis and for age an independent t-test.
Comparisons of the treatment groups with respect to baseline organoleptic scores were performed using an independent t-test. Within-treatment comparisons of the baseline versus follow-up organoleptic scores were performed using paired t-tests. Comparisons of the treatment groups with respect to baseline-adjusted organoleptic scores at the follow-up examinations were performed using analysis of covariance (ANCOVA). All statistical tests of hypotheses were two-sided and employed a level of significance of α = 0.05.
Results
Consumer Testing
In consumer studies in the United States (n = 164) and the United Kingdom (n = 150), participants preferred the flavor and freshening attributes of the new SNaP toothpaste over the in-market formulation of Colgate Total. They also indicated that this new toothpaste delivered better health-related attributes of "providing long-lasting protection" and "allowing me to be proactive about my oral health." The new formulation was parity on foaming and consistency attributes. Sixty percent of US consumers and 45% of UK consumers indicated a "5" (top score of agreement) regarding the new SNAP toothpaste's freshening attributes; freshness perception and long-lasting fresh breath were rated statistically significantly higher for the new formulation (90% confidence level [CL], two-tailed) compared to the in-market formulation of Colgate Total in both the United States and United Kingdom.
Malodor Reduction
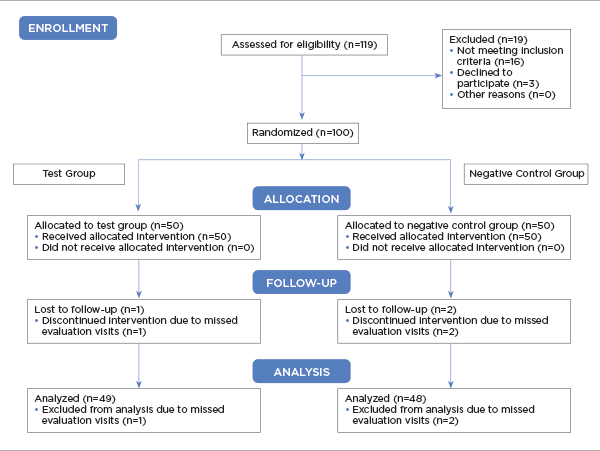
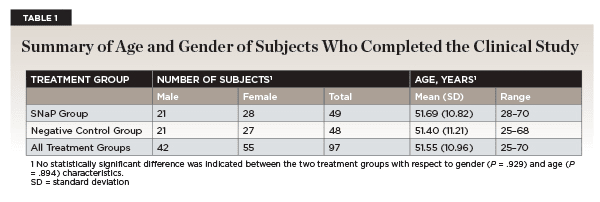
Ninety-seven subjects complied with the protocol and completed the clinical investigation (Figure 1). Three subjects failed to attend all the evaluations and were excluded from the study. The reasons were not product-related. A summary of the gender and age of the study population is presented in Table 1.
For the organoleptic oral malodor assessment, the mean baseline scores were 7.22 for subjects assigned to the SNaP toothpaste group and the negative control group. No statistically significant (P = .994) difference was indicated between the treatment groups with respect to organoleptic scores at baseline.
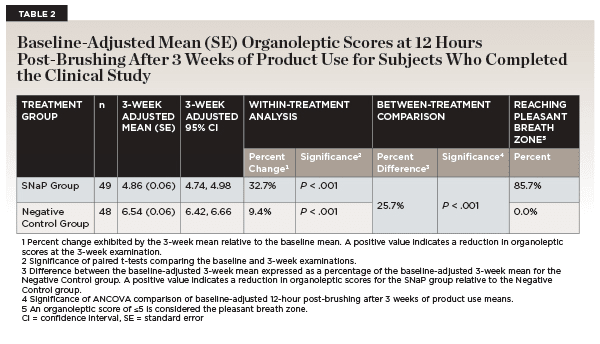
The baseline-adjusted mean (95% confidence interval [CI]) organoleptic scores evaluated 12 hours post-brushing were 4.86 (95% CI [4.74, 4.98]) for subjects assigned to the SNaP toothpaste group and 6.54 (6.42, 6.66) for subjects assigned to the negative control group (Table 2, Figure 2). The percent reductions from baseline were 32.7% for the SNaP toothpaste group and 9.4% for the negative control group. The percent reductions from baseline observed for the SNaP toothpaste group and negative control group were statistically significant (P < .001).
Use of SNaP toothpaste over a 3-week period provided a statistically significantly greater level of efficacy in controlling oral malodor as compared to the negative control toothpaste. Subjects who brushed with the SNaP toothpaste exhibited a 25.7% reduction in malodor versus the negative control group (P < .001), evaluated 12 hours post-brushing, after 3 weeks of product use via organoleptic scores (Table 2).
Additionally, 85.7% (42 out of 49) of the subjects who brushed with the SNaP toothpaste went into the pleasant breath zone (organoleptic score ≤5) after 3 weeks of product use, while 0.0% (0 out of 48) of the subjects who brushed with the negative control toothpaste containing 0.76% sodium monofluorophosphate did.
Adverse Events
No adverse events were observed by the investigator or reported by subjects.
Discussion
In this randomized clinical study, SNaP toothpaste showed improved malodor reduction benefits compared to the negative control group. Results indicate that the SNaP toothpaste formulation has the ability to reduce malodor by addressing the bacteria biofilm source of malodor. The results demonstrated that the use of SNaP toothpaste over a 3-week period provided a statistically significant greater level of efficacy in controlling malodor for up to 12 hours post-brushing as compared to a commercially available control product. Notably, by the end of the study, more than 85% of participants using SNaP toothpaste had pleasant breath even after 12 hours overnight. Results are consistent with the findings of a recent study testing the efficacy of dentifrices containing stannous fluoride to reduce volatile sulfur compounds, a type of metabolite particularly associated with halitosis.17 In alignment with the pleasant breath results of the clinical trial, consumer testing also indicated an appealing perception of long-lasting freshness. This could be due to the stannous stabilization system of the SNaP toothpaste.
Stannous fluoride has been found effective in its ability to fight bacteria, including those that produce malodorous compounds.8-10 Trial results indicate SNaP toothpaste's efficacy to reduce malodor, but clinical benefit cannot be delivered to consumers without consistent use. Flavor is a driver of consumer behavior for toothpaste use. The appealing flavor for the SNaP dentifrice base was preferred by consumers in the United States and the United Kingdom.
The clinical evaluation was a well-controlled, randomized, double-blind trial. It took place, however, at a single site with a specific patient population that suffers from halitosis. Results may not translate to all patient groups but may be highly relevant to other patient groups with halitosis. Future research in certain key areas could enhance the dental profession's understanding and treatment of oral malodor. Comprehensive studies evaluating the long-term efficacy of different treatments could shed light on the sustainability of their results over time. Identifying specific bacteria and bodily processes, as well as the biological mechanisms behind oral malodor, could aid in the development of more targeted and effective treatments. Lastly, performing comparative studies to assess the efficacy of different treatments across various types of oral malodor (physiological and pathological) could help determine the most effective solutions for each specific condition.
Conclusions
The SNaP formulation with the winning flavor was shown to deliver significant malodor reduction benefits as compared to a negative control toothpaste. Based on the results of the consumer and clinical studies, the SNaP formulation delivers positive esthetic and therapeutic benefits, which are essential to support consumers' everyday use and deliver the benefits of whole-mouth health.
ACKNOWLEDGMENTS
Technical writing was provided by Cynthia Drake Morrow, PhD, MA, and Jennifer Wisdom, PhD, MPH, ABPP. The author contributions were as follows: MB and JD: investigation, methodology; AC: investigation, methodology, project administration; DH: investigation, methodology, resources; LM: formal analysis; MR: conceptualization, funding acquisition; YZ: conceptualization, supervision, funding acquisition. All authors, including TB, CM, RD, and PM, contributed to writing, review, and editing.
DISCLOSURES
This clinical trial was supported by a grant by Colgate-Palmolive Company. ClinicalTrials.gov: NCT06300905. The study was reviewed and approved by the Institutional Review Board of China Oral Health Foundation, 18-A South Avenue, Zhongguancun, Haidian District Beijing, 100081. The authors AC, MB, JD, TB, CM, PM, RD, MR, and YZ are employees of Colgate-Palmolive Co. DH is a clinical investigator with no conflicts of interest to declare. LM is an independent statistical consultant to Colgate-Palmolive Co.
DATA AVAILABILITY
The documents containing the results of the research herein described are confidential. The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
About the Authors
Aileen Cabelly, MS
Director Technology Insights Oral Care, Colgate-Palmolive Co., Piscataway, New Jersey
Mania Bankova, PhD
Director Research and Development (R&D), Colgate-Palmolive Co., Piscataway, New Jersey
Jason Darling, BS
Senior Director Flavors R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Tracy Bariexca, BS
Senior Principal Scientist R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Catalina Monroy
Senior Vice President Flavors and Fragrances, Colgate-Palmolive Co., Piscataway, New Jersey
Deyu Hu, DDS, MS
Professor, West China Dental Institute of Chengdu, Sichuan, China
Luis R. Mateo, MA
President, LRM Statistical Consulting LLC, West Orange, New Jersey
Pamela Monty, BS, RDH
Clinical Research Specialist, Colgate-Palmolive Co., Piscataway, New Jersey
Robert D'Ambrogio, BS
Senior Principal Scientist R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Maria Ryan, DDS, PhD
Executive Vice President, Clinical Research, Knowledge Management and Scientific Communications, Colgate-Palmolive Co., Piscataway, New Jersey
Yun-Po Zhang, PhD, DDS (Hon)
Senior Vice President and Distinguished Fellow, Clinical Research, Colgate-Palmolive Co., Piscataway, New Jersey
References
1. Dadamio J, Laleman I, Quirynen M. The role of toothpastes in oral malodor management. Monogr Oral Sci.2013;23:45-60.
2. Wu J, Cannon RD, Ji P, et al. Halitosis: prevalence, risk factors, sources, measurement and treatment - a review of the literature. Aust Dent J. 2020;65(1):4-11.
3. Olszewska-Czyz I, Sozkes S, Dudzik A. Clinical trial evaluating quality of life in patients with intra-oral halitosis. J Clin Med.2022;11(2):326.
4. Tsuruta M, Takahashi T, Tokunaga M, et al. Relationships between pathologic subjective halitosis, olfactory reference syndrome, and social anxiety in young Japanese women. BMC Psychol. 2017;5(1):7.
5. Hampelska K, Jaworska MM, Babalska ZL, Karpinski TM. The role of oral microbiota in intra-oral halitosis. J Clin Med. 2020;9(8):2484.
6. Foo LH, Balan P, Pang LM, et al. Role of the oral microbiome, metabolic pathways, and novel diagnostic tools in intra-oral halitosis: a comprehensive update. Crit Rev Microbiol.2021;47(3):359-375.
7. Aung EE, Ueno M, Zaitsu T, et al. Effectiveness of three oral hygiene regimens on oral malodor reduction: a randomized clinical trial. Trials. 2015;16:31.
8. Myers CP, Pappas I, Makwana E, et al. Solving the problem with stannous fluoride: formulation, stabilization, and antimicrobial action. J Am Dent Assoc. 2019;150(4S):S5-S13.
9. Haraszthy VI, Raylae CC, Sreenivasan PK. Antimicrobial effects of a stannous fluoride toothpaste in distinct oral microenvironments. J Am Dent Assoc. 2019;150(4S):S14-S24.
10. Chakraborty B, Triratana SD, Mateo LM, et al. Antibacterial effects of a novel stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP): in vitro study and randomized controlled trial. Compend Contin Educ Dent. 2024;45 suppl 3:12-20.
11. Patel A, Amrit S. Formulation taste masking-from bitter to better: the latest taste masking techniques can yield more palatable drugs. Pharmaceutical Formulation and Quality. 2009:1-2.
12. Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm.2004;30(5):429-448.
13. Zhang S, Govindaraju GV, Cheng CY, et al. Oxidative stability of chelated Sn(II)(aq) at neutral pH: the critical role of NO3− ions. Sci Adv.2024;10(40). doi: 10.1126/sciadv.adq0839.
14. Lee S, Li Y, Mateo LR, et al. A 6-month randomized controlled trial to measure the efficacy of a stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) on dental plaque and gingivitis. Compend Contin Educ Dent. 2024;45 suppl 3:21-29.
15. Elias-Boneta AR, Mateo LR, D'Ambrogio R, et al. Efficacy of a novel stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) in extrinsic tooth stain removal: a randomized controlled trial. Compend Contin Educ Dent. 2024;45 suppl 3:46-52.
16. Liu Y, Lavender S, Ayad F, et al. Effect of a stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) on dentin hypersensitivity: in vitro study and randomized controlled trial. Compend Contin Educ Dent.2024;45 suppl 3:30-39.
17. Zsiska M, Schneiderman E, Jin Y, et al. Investigation of oral malodor prevention by dentifrices as measured by VSC reduction. J Breath Res. 2021;15(3). doi: 10.1088/1752-7163/abf209.