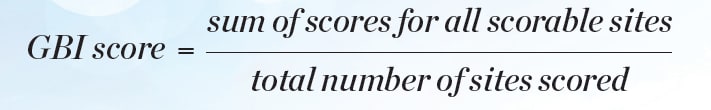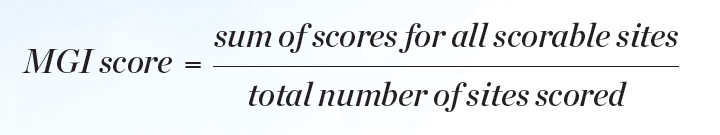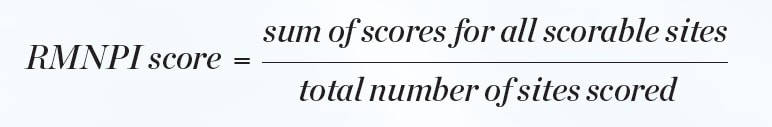Kimberly R. Milleman, RDH, PhD; Jeffery L. Milleman, DDS, MPA; Megan Gaff, BSDH; Kristina Cook, MASc; and Luis Mateo, MSc
ABSTRACT: Objective: To evaluate the safety and efficacy of the Oral Health System by Fresh Health Inc., used in conjunction with manual toothbrushing (Fresh + MTB) as compared to string floss and manual toothbrushing (floss + MTB) and manual toothbrushing (MTB) alone, as measured by clinical signs of gingivitis, plaque reduction, pocket depth, and bleeding. Methods: One hundred ninety-two (192) generally healthy adults exhibiting signs of gingivitis completed this 30-day randomized, controlled, examiner-blinded, three-group parallel study design. All subjects were assigned a manual toothbrush and fluoride dentifrice, instructed to brush twice daily according to their normal habits, and provided with written and verbal instructions for all assigned products. Subjects in the control group used only the manual toothbrush and dentifrice. Subjects assigned to the string floss + MTB group were instructed to also floss once daily. Subjects assigned to the Fresh + MTB group were provided a Fresh Health Inc. custom-fit oral irrigator and instructed to use the device once daily with water for approximately 7 seconds in addition to toothbrushing. Gingivitis was assessed using the modified gingival index (MGI), bleeding on marginal probing was assessed via the gingival bleeding index (GBI), and plaque was measured using the Rustogi modified navy plaque index (RMNPI) at day 1, day 15, and day 30. Periodontal probing depth (PPD) and bleeding on probing (BOP) were measured at day 1 and day 30. Oral soft- and hard-tissue assessments were performed at all examination visits. Results: There was no significant difference in age or sex between groups, and no significant difference in baseline MGI, GBI, RMNPI, BOP, and PPD values across groups. The Fresh + MTB group demonstrated statistically significantly better performance than the floss + MTB group and MTB group across all clinical indices at both 15 days and 30 days. At 30 days, the Fresh + MTB group showed a 40.9% improvement in whole-mouth MGI, which was significantly greater than the MTB and floss + MTB groups (P < .001). At 15 days and 30 days, the Fresh + MTB group showed 74% and 82% reduction in interproximal bleeding, respectively, significantly greater than the MTB and floss + MTB groups (P < .001). Significant 30-day improvements as compared to MTB and floss + MTB groups were also observed for RMNPI (P < .001), PPD (P < .001), and BOP (P = .001). Conclusions: Subjects who were assigned to the Fresh + MTB group showed significantly greater reductions in gingival inflammation, gingival bleeding, plaque accumulation, BOP, and pocket depth measurements than those in the MTB and floss + MTB groups.
Periodontal diseases are highly prevalent globally. Studies have shown that close to 94% of adults in the United States show signs of gingivitis.1 Gingivitis occurs as a result of an inflammatory response to microbial biofilms, ie, the bacteria in dental plaque, and leads to inflammation and bleeding of the gingiva. Left untreated, gingivitis may progress to a more severe form of periodontal disease: periodontitis. The Centers for Disease Control and Prevention has reported that in the United States, 42.2% of the population aged 30 or older and 70.1% of the population aged 65 or older has periodontal disease.2 Gingival inflammation in response to dental plaque is recognized as the key risk factor for development of periodontitis3-5 and tooth loss,6 and as such, the control of gingival inflammation is critical for the primary prevention of periodontitis. Further highlighting the importance of maintaining good periodontal health is the increase in emerging data showing associations between periodontal disease and systemic diseases.7-9
Plaque-induced gingivitis, however, is reversible, and improving oral hygiene is an effective means to reduce and prevent gingivitis. Although numerous products currently exist on the market that aim to reduce and disrupt plaque and reduce and prevent gingivitis, compliance is lacking. Recent reviews demonstrate that current interdental oral hygiene measures such as string floss, oral irrigators, interdental brushes, and wooden picks offer varying and inconsistent levels of success and may be difficult to use effectively.10 The complexity and time required to use string floss has been cited as a reason for reduced compliance,11 and studies have shown that few individuals floss correctly.12 Systematic reviews have demonstrated that oral irrigators currently on the market, used as an adjunct to brushing, have a beneficial effect on clinical parameters of periodontal inflammation, although a benefit in removing visible plaque has not been consistently demonstrated.13,14
Increasingly, the complexity of oral biofilm and the importance of supporting bacterial homeostasis has been reported,15 with the ultimate goal of supporting a healthy and balanced oral microbiome. Interdental spaces have notoriously been difficult to access and clean appropriately, which is critical to the success of an interdental cleaning aid.16 There remains a need for a fast, efficient, safe, and easy-to-use device to aid in reduction/prevention of gingivitis and in the disruption/reduction of plaque.
This study evaluates a novel oral care product, the Oral Health System by Fresh Health Inc. The system aims to remove the variability and challenges associated with traditional interdental cleaners by delivering pulsed fluid through custom interproximally positioned nozzles in a custom-fabricated mouthpiece in as little as 7 seconds daily. The objective of this human clinical study was to evaluate the safety and efficacy of the Fresh System plus manual brushing compared to manual brushing alone and manual brushing plus string floss to reduce clinical signs of gingivitis and dental plaque accumulations.
Methods and Materials
Ethical Aspects
Prior to subject recruitment, the study was approved by an independent Institutional Review Board (US IRB Miami, FL 33143; IRB number U.S.IRB2022SRI/06). This study was conducted in accordance with good clinical practice, the Declaration of Helsinki, and local and federal regulations. Subjects were recruited by Salus Research, Inc. (Fort Wayne, Indiana), an American Dental Association (ADA)-qualified research site, and written informed consent was obtained from all subjects prior to enrollment in the study.
Study Population
Subjects were generally healthy adults, aged 18 or older, with at least 20 natural scorable teeth and at least one first or second premolar or first molar present in each quadrant. Qualifying subjects, at baseline, had at least 50 bleeding sites (gingival bleeding index [GBI] score of 1 or 2), a whole-mouth modified gingival index (MGI) mean score of at least 1.75, and a pre-brushing whole-mouth Rustogi modified navy plaque index (RMNPI) mean score of 0.6.
Exclusion criteria included current or recent (<4 weeks) participation in another clinical study; extreme crowding or overlapping of teeth; hard- or soft-tissue lesions; aggressive, necrotizing, or other uncommon periodontal disease states; current or previous (<6 weeks) therapy with medications or antibiotics or systemic condition or infectious disease that may interfere with the outcome of the study; active tobacco users; extensive calculus that may interfere with scoring of the teeth; extreme tooth or gum sensitivity; fixed or removable orthodontic appliances or extensive restorations; and females who were pregnant or lactating. The presence of dental implants was not an exclusion criterion; however, none of the subjects had implants. Subjects agreed to avoid elective dental procedures and refrained from using oral care products other than those assigned to them for the duration of the study.
Clinical Assessment
Subjects were evaluated by blinded examiners, who were dental hygienists. The same blinded examiner assessed oral soft- and hard-tissue health, gingival inflammation, gingival bleeding on marginal probing, and dental plaque at each study visit, and examiners underwent intraexaminer calibration prior to the start of the study. A second blinded examiner performed pocket depth measurements and recorded bleeding on probing (BOP) at both the baseline and day 30 visits. At each examination visit, all subjects were asked about any changes in health or medications that occurred during the study. Subjects refrained from performing oral hygiene for 12 to 18 hours prior to clinical assessments and were asked to refrain from eating or drinking 30 minutes prior to examinations.
Gingivitis was evaluated using the MGI followed by the GBI.17,18 For both measures, six sites per tooth (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, and distolingual) were scored: the distal and mesial regions of interdental papilla and the buccal and lingual marginal gingival regions. MGI was recorded on a scale from 0 (absence of inflammation) to 4 (severe inflammation). GBI was recorded on a scale from 0 (no bleeding) to 2 (extreme bleeding) as noted within 30 seconds of marginal probing.
Supragingival plaque was disclosed using a plaque-disclosing solution (Trace® Disclosing Solution, Young Dental, youngdental.com) and was recorded as present or absent on nine discrete areas of the tooth on both the facial and lingual surfaces according to the RMNPI.19
Periodontal probing depth (PPD) measurements (mm) and BOP were recorded at six sites per tooth (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, and distolingual), with BOP recorded as present or absent.
MGI, GBI, and RMNPI were measured at all visits (screening, day 1, day 15, and day 30); PPD and BOP were measured at day 1 and day 30. Measurements were recorded for all scorable teeth, excluding third molars.
Investigational Products
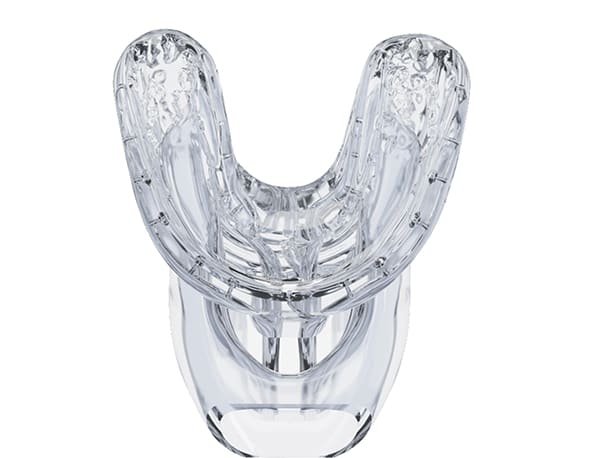
This clinical trial investigated the efficacy of a novel oral irrigation device (Oral Health System by Fresh Health Inc., freshhealth.com) (Figure 1). The system delivers pulsatile pressurized fluid through precision positioned nozzles in a custom-fabricated 3D-printed mouthpiece (Figure 2 and Figure 3). Up to 60 nozzles are positioned at facial and lingual interproximal sites, as determined by intraoral scans. Treatment is delivered via the press of a button.
All subjects were provided with the same ADA reference manual soft toothbrush and ADA accepted, 0.243% sodium fluoride dentifrice (Crest® Cavity Protection, Procter & Gamble, us.pg.com). Subjects assigned to the string floss group (floss + manual toothbrushing) were provided with ADA accepted readily available string floss. Subjects assigned to the Fresh group were provided with their custom 3D-printed mouthpiece. Custom mouthpieces were designed and manufactured by Fresh Health Inc.
Study Design
This investigation used a randomized, controlled, examiner-blind parallel study design to compare MGI, GBI, RMNPI, PPD, and BOP across three treatment groups: Group 1 - once-daily use of the Oral Health System by Fresh Health Inc. with water in conjunction with twice-daily manual toothbrushing (Fresh + MTB); Group 2 - once-daily string floss use in conjunction with twice-daily manual toothbrushing (floss + MTB); Group 3 - twice-daily manual toothbrushing only (MTB).
At the screening visit, subjects were evaluated to ensure they would qualify for the trial by presenting with sufficient gingival inflammation as measured by the MGI and GBI and supragingival plaque as per the RMNPI. Medical history and demographic information were collected. Qualifying subjects were then randomized into the three groups.
Qualifying subjects randomized to the Fresh + MTB group had digital intraoral scans performed (TRIOS 3, 3Shape, 3shape.com) with their arch position recorded. Custom 3D-printed oral irrigation devices were produced by Fresh Health Inc. based on the subject's oral anatomy, as captured by the intraoral scan data.
At baseline (day 1), all subjects received a pre-brushing oral examination and assessment to ensure they continued to meet the minimum MGI, GBI, and RMNPI requirements. Examinations occurred in the following order: oral examination of hard and soft tissues, MGI, GBI, and RMNPI after timed disclosing with 10 mL of plaque-disclosing agent. Subjects then had PPDs measured, followed by recording of the presence or absence of BOP.
After baseline examinations, subjects were provided with study products and written and verbal instructions in a private area to ensure blinding of examiners. All subjects were instructed to brush their teeth twice daily at home according to their usual habits, once in the morning and once in the evening, using their assigned toothbrush and toothpaste over the 30-day study period. Subjects assigned to the floss + MTB group were provided with their assigned string floss and instructed to floss once daily at home. In addition, subjects in this group were provided with the "ADA How to Floss Pamphlet" and given verbal instructions, and they performed their first daily flossing under direct supervision by the site research staff. Subjects assigned to the Fresh + MTB group were provided with their custom-made mouthpiece for use at the research site. Subjects were provided with a Quick Start Guide from the manufacturer, and were given verbal instructions. The Fresh + MTB group used the Fresh System once daily at the research site with water; the product was set to "maximum" and delivered approximately 7 seconds of treatment. Product use was recorded in each subject's treatment diary/log.
At the interim visit (day 15 +/- 2 days) and final visit (day 30 +/- 2 days), all subjects returned for their oral examination of hard and soft tissues, followed by MGI, GBI, and RMNPI evaluations, in that order (followed by PPD and BOP evaluations for the 30-day visit only). Subjects did not perform oral hygiene 12 to 18 hours prior to their examinations and refrained from eating or drinking 30 minutes prior to their examinations.
Visual examination of the oral cavity was conducted using a dental light and mirror. The structures examined included the gingivae, hard and soft palates, oropharynx, buccal and labial mucosa, tongue, floor of the mouth, and lips, as well as tooth crowns and exposed roots and cervical areas. The site, size, and severity of any lesions and tentative diagnosis, if possible, were recorded, and restorations were assessed for any damage. A judgment was made by the examiner as to whether or not any aberrations were attributable to the test product based on the experience of the principal investigator and dental examiner with these types of studies. Any new abnormal findings recorded after product distribution were recorded as adverse events. No adverse events were reported during the study. Dentifrice and string floss were weighed at day 1 and day 30 to estimate compliance, and subject diaries were reviewed.
Sample Size and Statistical Analysis
The sample calculation was performed using data from a previous pilot study. The planned sample size of n = 210 (70 subjects per group) for this clinical trial was determined based on the pooled standard deviation(s) of ≤0.09, ≤0.25, and ≤0.33 units, respectively, for the two primary response measure(s), bleeding on marginal probing (BOMP) via GBI and MGI, and the secondary response measure, RMNPI. The overall sample size of n = 210 provided a level of power of 90% to detect a minimal statistical difference among the study group means of 0.54, 0.30, and 0.22 units, respectively, for GBI, MGI, and RMNPI with a two-sided significance α-level of 0.05 and an attrition rate of 15%.
Statistical analyses for efficacy in gingivitis reduction were performed based on change-from-baseline-adjusted whole-mouth (and interproximal) GBI scores and MGI scores. BOMP via GBI and MGI scores was computed for each subject, and each tooth surface was scored in six areas. Whole-mouth GBI and MGI within subject mean scores were computed at each clinical examination (baseline, interim, and final visits) as shown in Equation 1 and Equation 2.
The whole-mouth data (six sites per tooth) and all interproximal regions (four sites per tooth) were analyzed. Third molars were excluded from scoring.
The secondary efficacy outcome variables of the study were the plaque index scores as measured by the RMNPI among the study groups. Additional secondary efficacy outcome variables of the study were PPD (mm) and BOP scores at each of the six tooth sites.
Supragingival plaque on the facial and lingual surfaces of each tooth was recorded as present or absent on nine discrete areas of each scorable tooth. Third molars were excluded from the scoring procedure. From these site-wise scores, a whole-mouth score was determined for each subject by calculating the proportion of sites in the mouth at which plaque was present (Equation 3).
The whole-mouth data (nine sites on each facial/lingual surface per tooth), gingival margin regions (three sites per tooth on both facial/lingual surfaces), and interproximal regions (two sites per tooth on both facial/lingual surfaces) were analyzed.
A chi-square test was performed on the sex demographic data to evaluate the hypothesis that the treatment groups were randomized and balanced with respect to sex. In addition, an analysis of variance (ANOVA) was performed on the age demographic data to evaluate the hypothesis that the mean age of the treatment groups was balanced with respect to age.
Descriptive statistics (mean, standard deviation [SD]) were computed for GBI, MGI, and RMNPI scores at each timepoint for each treatment group, as applicable. Comparisons of baseline GBI, MGI, and RMNPI scores were made using an ANOVA. Within-treatment group comparisons between baseline and post-treatment scores were performed using paired t-tests. Analyses of covariance (ANCOVAs) were employed on the post-treatment or delta scores to compare the study treatment groups with respect to each of the clinical parameters (GBI, MGI, and RMNPI) at the post-baseline timepoints using the baseline measure as the covariate. If the F-test of the ANCOVA exhibited a statistically significant difference among the treatment groups at the 0.05 significance level, then post-ANCOVA pair-wise comparisons of the treatment groups were performed using Tukey's test for multiple comparisons. All statistical tests of hypotheses were two-sided and employed a level of significance of α = 0.05.
Descriptive statistics were computed for PPD (mm) and BOP scores at each timepoint for each treatment group. Statistical analyses were performed on the PPD (mm) and BOP for each of the six tooth sites assessed. Comparisons of the treatment groups with respect to the baseline PPD (mm) and BOP scores were assessed for each of the six tooth sites using an ANOVA. Within-treatment group comparisons of the baseline versus follow-up PPD (mm) and BOP scores for each of the six tooth sites assessed were performed using paired t-tests. Comparisons of the treatment groups with respect to baseline-adjusted PPD (mm) and BOP scores at the follow-up examinations were performed using ANCOVAs for each of the six tooth sites assessed. All statistical tests of hypotheses were two-sided and employed a level of significance of α = 0.05.
Results
Study Population
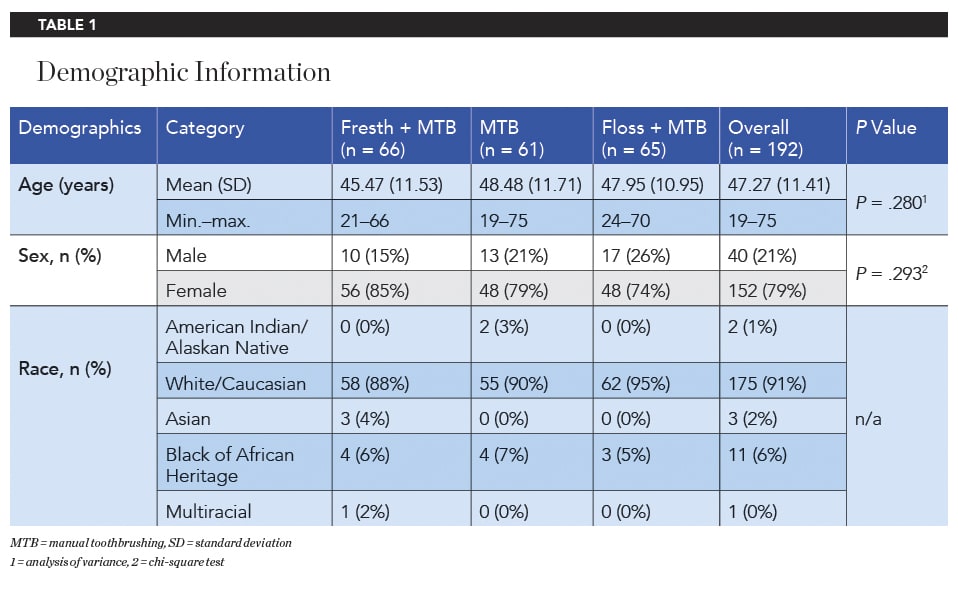
Of 219 total recruited subjects, two were screening/baseline failures, and 217 qualified and were enrolled into the study. Subjects who dropped out prior to the baseline examination were replaced, if possible. From among 195 subjects that received product at baseline, 192 subjects completed the study. The study population consisted of 152 females and 40 males, with a mean age (SD) of 47.27 (11.41) years (Table 1). There was no significant difference in age and sex between the three treatment groups.
Gingivitis Reduction Efficacy
At baseline, there was no significant difference in MGI or GBI between groups, both across the whole mouth (six sites per tooth, MGI P = .332, GBI P = .596) and all interproximal sites (four sites per tooth, MGI P = .146, GBI P = .499).
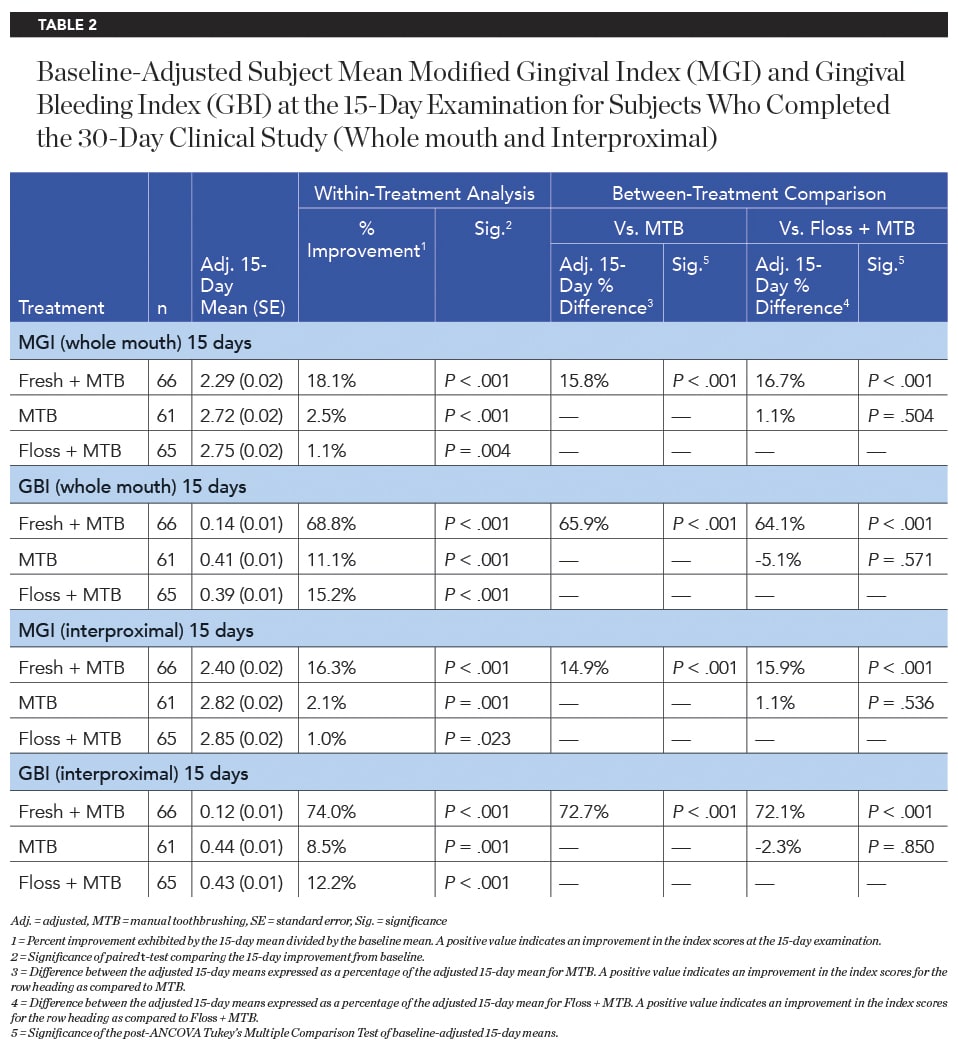
After 15 days of product use, the Fresh + MTB group showed a significantly greater improvement in MGI and GBI than the MTB group and floss + MTB group, both interproximally and across the whole mouth (Table 2). The Fresh + MTB group showed 16.5X greater improvement in whole-mouth MGI and 4.5X greater improvement in whole-mouth bleeding on marginal probing via GBI than the floss + MTB group. Improvement in bleeding was more pronounced when assessing interproximal sites, with the Fresh + MTB group showing a 74% reduction in interproximal bleeding in 15 days, 6.1X as effective as the floss + MTB group.
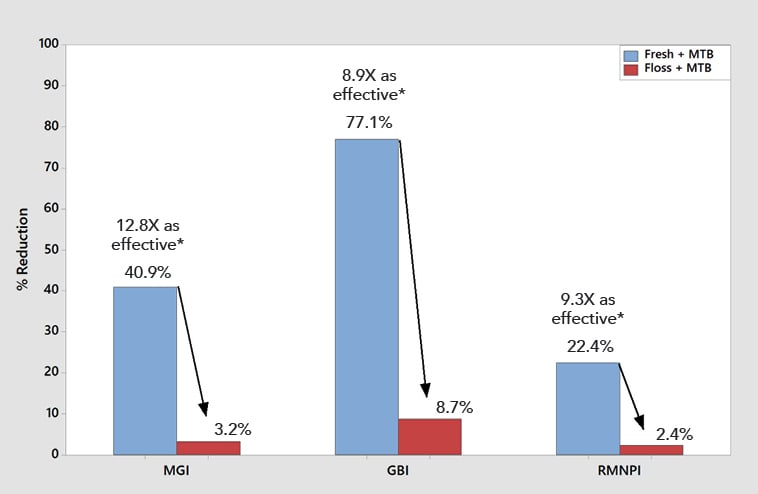
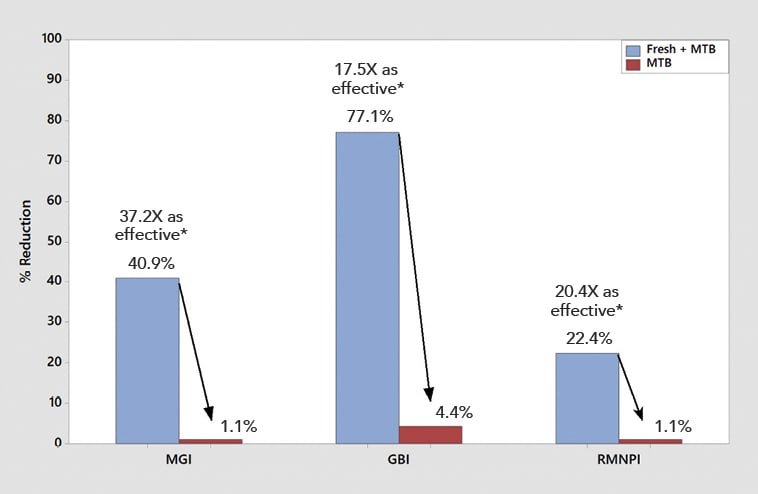
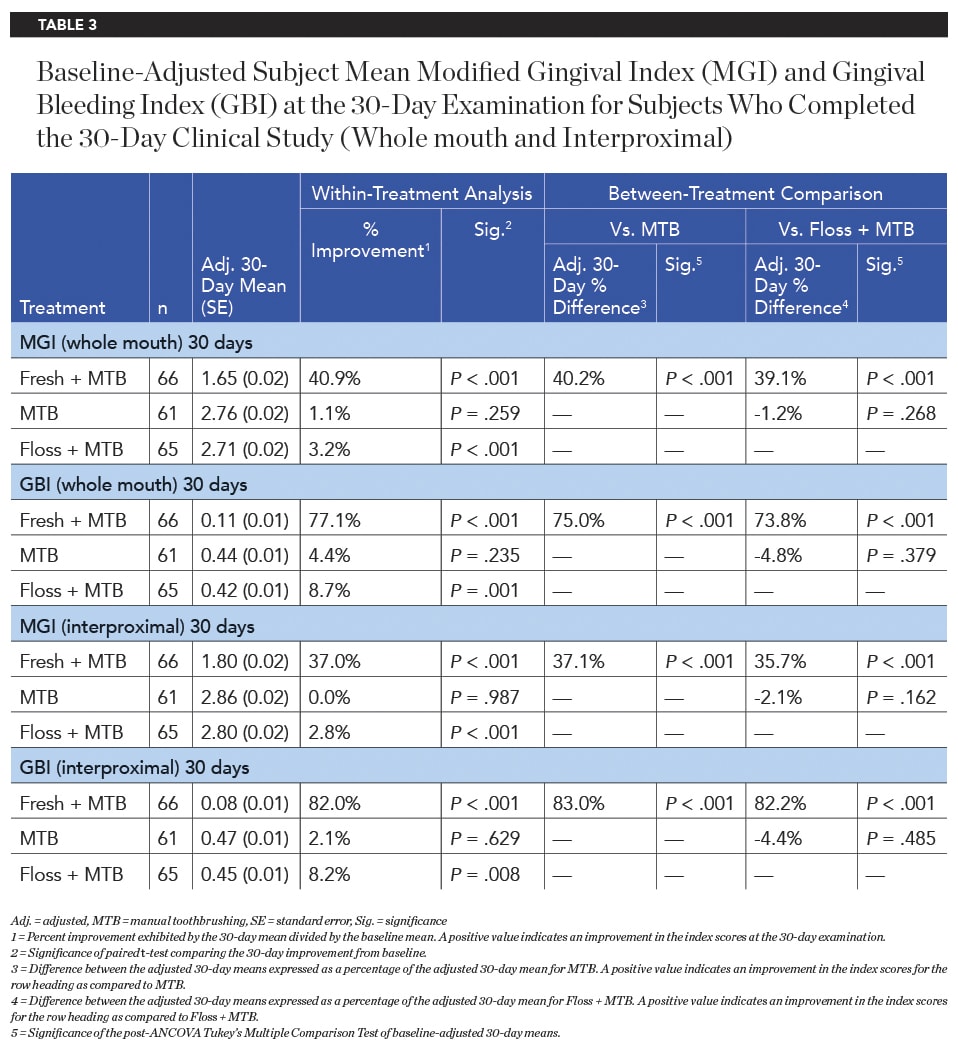
Significantly superior improvement in gingivitis outcomes continued for the Fresh + MTB group at the 30-day examination, with this group showing a 40.9% improvement in MGI (12.8X times as effective as floss + MTB) and a 77.1% improvement in GBI (8.9X as effective as floss + MTB) across the whole mouth (Table 3, Figure 4). Significantly superior improvements in gingivitis outcomes were also demonstrated for the Fresh + MTB group as compared to the MTB group (Table 3, Figure 5). Interproximally, the reduction in bleeding was more notable, at 82% improvement in 30 days for the Fresh + MTB group, 10X as effective as the floss + MTB group.
Plaque Reduction
No statistically significant difference was observed among the treatment groups at baseline with respect to RMNPI (P = .399), proximal RMNPI (P = .400), or gingival margin RMNPI (P = .341) indices.
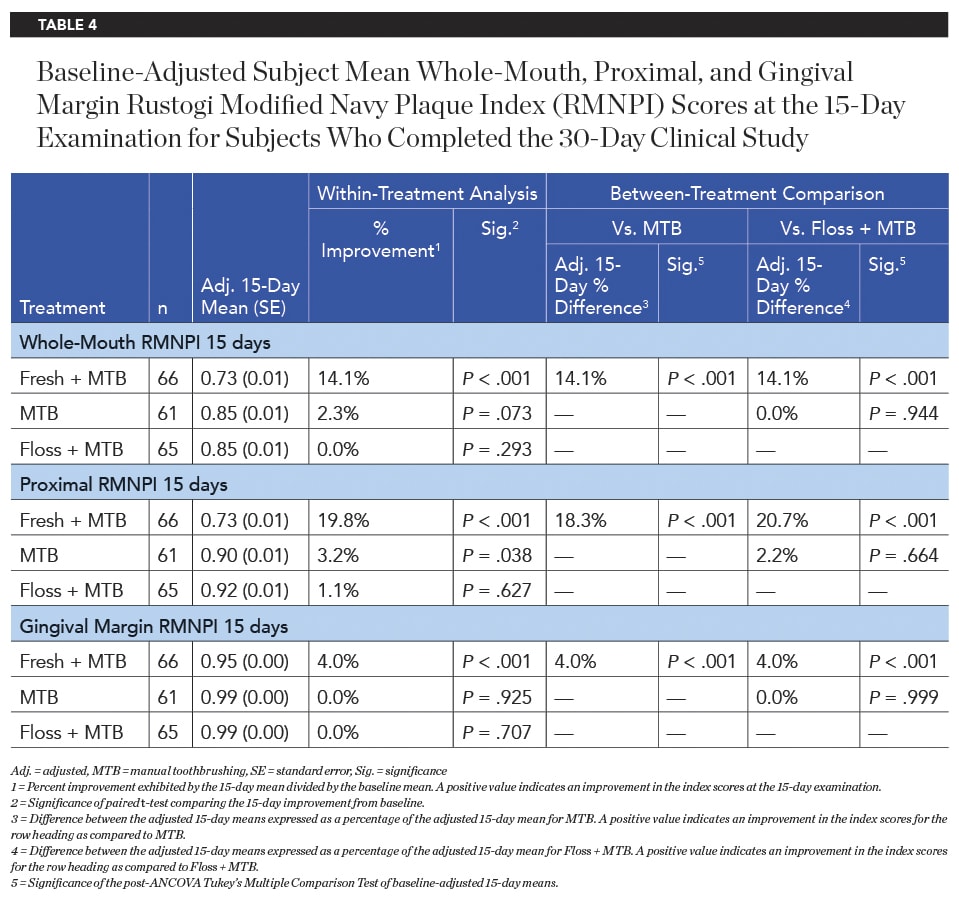
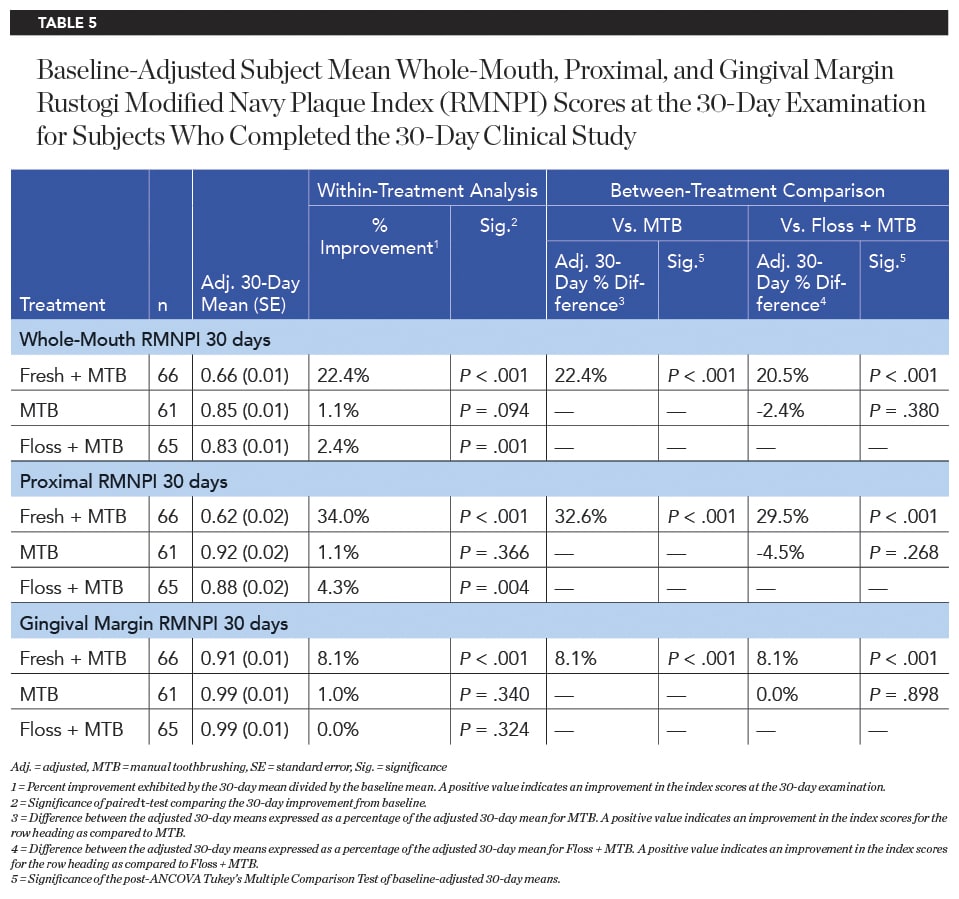
After 15 days of product use, the Fresh + MTB group showed a significantly higher improvement in plaque reduction than both the MTB group and floss + MTB group for whole-mouth, proximal, and gingival margin regions (Table 4). This trend continued after 30 days of product use, with the Fresh + MTB group showing a 22.4% reduction in plaque, a 9.3X greater reduction than the floss + MTB group (Table 5).
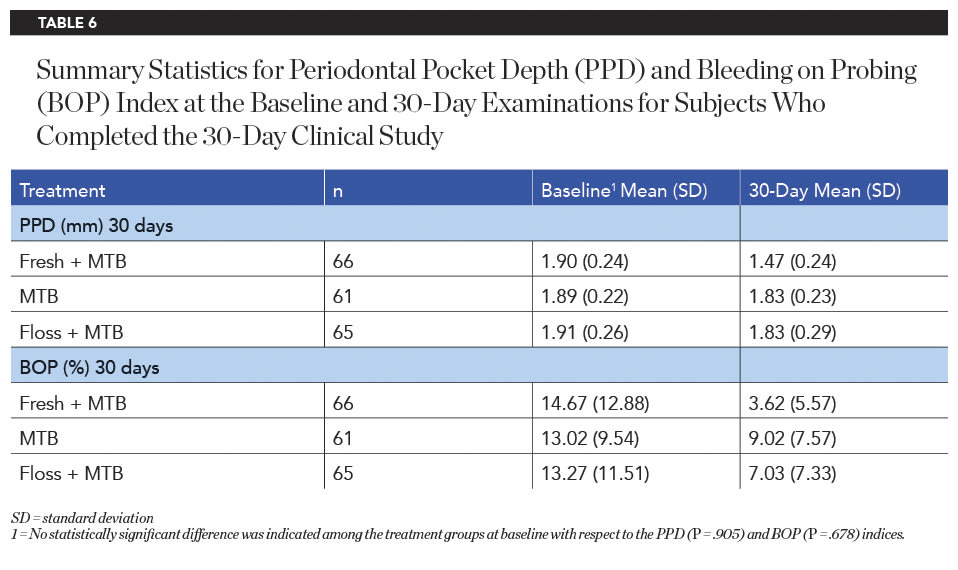
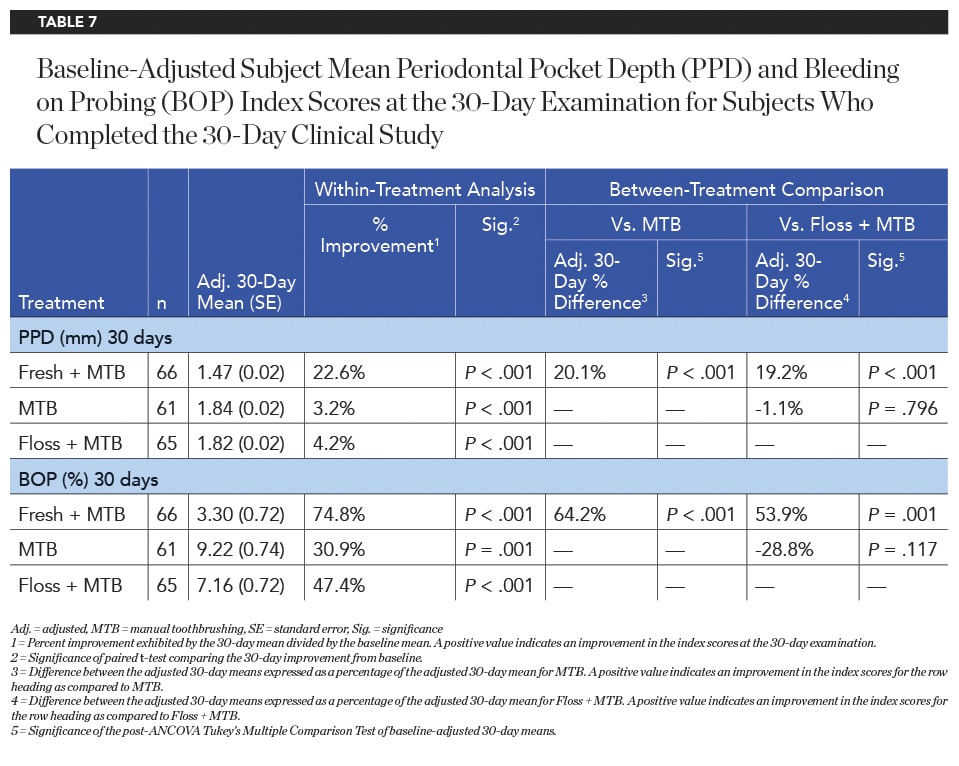
Pocket Depth and Bleeding on Probing
The baseline periodontal probing depth (PPD) and bleeding on probing (BOP) values did not differ significantly between groups (Table 6). After 30 days of product use, the Fresh + MTB group demonstrated statistically significantly greater reductions in PPD compared to both the MTB and floss + MTB groups (Table 7). On average, subjects in the Fresh + MTB group showed a reduction of 0.43 mm in pocket depth over 30 days of product use, as compared to 0.06 mm and 0.08 mm for the MTB and floss + MTB groups, respectively. Bleeding within the sulcus, as measured by BOP, also favored the Fresh + MTB group, which showed significantly more reduction in bleeding than both the MTB and floss + MTB groups (Table 7).
Discussion
This study demonstrated that once-daily 7-second use of the Oral Health System by Fresh Health Inc. with water in conjunction with manual toothbrushing was statistically significantly more effective at reducing gingival inflammation, gingival bleeding, and supragingival plaque than use of a manual toothbrush and string floss or a manual toothbrush alone over 15-day and 30-day periods in patients with gingivitis. Additionally, the Fresh System used in conjunction with manual toothbrushing was significantly more effective than both manual toothbrushing alone and use of string floss in conjunction with manual toothbrushing in reducing pocket depths and BOP (from within the sulcus) after 30 days. This reduction in pocket depth is likely due to the substantial reduction in swelling and inflammation (ie, pseudopockets) in the interproximal papillary area. The significant improvement in gingival health may be indicative of the Fresh System's ability to deliver fluid below the gingival margin to flush the gingival sulcus of biofilm and toxins associated with disease progression.
While the Fresh + MTB group did show significantly superior improvements as compared to the MTB and floss + MTB groups, it is worth noting that the MTB and floss + MTB groups also saw significant longitudinal improvements from baseline to 15 days for MGI and GBI. At 30 days, however, no significant difference was noted longitudinally in MGI or GBI for the MTB group. Although significant within-treatment improvements in MGI and GBI were noted at 15 and 30 days for the floss + MTB group, as were improvements in RMNPI, BOP, and PPD at 30 days, no significant difference in MGI, GBI, RMNPI, PPD, or BOP was noted between the floss + MTB and MTB groups, and the Fresh + MTB group performed significantly better than the MTB and floss + MTB groups across all measured clinical parameters (Table 4 and Table 5).
A possible explanation for the improvements in the MTB group is the introduction of required twice-daily brushing for all subjects; brushing frequency was not a condition for qualification in the study at screening. Moreover, the study population enrolled into this clinical trial exhibited at least 50 bleeding sites (using the GBI of up to 168 observation sites) according to the study protocol inclusion criteria. It was not surprising that all test subjects had improvements in their oral health during the clinical trial due to the requirement of at least moderate gingivitis at the baseline examination. During the trial period, subjects in all treatment groups brushed unsupervised at home with their assigned products twice daily. The Hawthorne effect and its potential to alter subjects' behavior undoubtedly occurred during the research study.20 Numerous toothbrushing and oral hygiene studies have reported initial reductions of gingivitis and plaque scores that have been attributed to the Hawthorne effect.21,22 This phenomenon can be directly linked to a behavior change where test subjects are aware that they are involved in a research study23 and increased attention bias or initial novelty effect is to be expected from them.24 Thus, the improvement in the control group (in a short-term study) is expected and any significant effect over the control group can be considered the relative efficacy of the treatment product.
The group using the Fresh System (Fresh + MTB group) displayed significant improvements in all measures across all timepoints when compared to the MTB and floss + MTB groups. The custom fit of the mouthpiece with precisely positioned nozzles delivering targeted fluid streams to both the lingual and facial aspects of the interdental spaces may be one reason for this, as human error in properly manipulating or positioning interdental oral hygiene tools during use is eliminated. A recent study evaluating the effect of manual dexterity on gingival bleeding and inflammation has shown that individuals with lower manual dexterity scores had less improvement (or worsening) in interproximal bleeding after 12 weeks of string floss and manual toothbrush use as compared to those with higher dexterity scores.25 The Fresh System removes the manual dexterity challenges associated with conventional string floss.
This study has potential limitations. Because dental floss is the interdental cleaner most commonly recommended by healthcare professionals,26 it was selected for comparison in this study. However, numerous adjuncts to brushing are available in the market. Future studies may compare the adjunctive benefit of other oral care products, including, for example, interdental brushes and oral irrigators. Additional opportunities for future research include evaluation in a more diverse population base, including those with different baseline levels of gingival and periodontal health as well as restorations that are challenging to clean. Additional research could also evaluate longer-term clinical outcomes and assess patient preferences and compliance.
Conclusion and Clinical Implications
The findings of this research study support the conclusion that the innovative Oral Health System by Fresh Health Inc. can provide superior oral health benefits, including statistically significant reductions in gingivitis, gingival bleeding, plaque, and pocket depths, as it did in this 30-day research study. Additionally, the patient-matched oral irrigator delivers a precise amount of water spray to hard-to-reach interdental areas of the mouth in a manner that is simple and quick to perform.
ACKNOWLEDGMENT
The authors thank the staff at Salus Research, Inc., including Abbie Yoder, BS, for her support as clinical coordinator and Kaylie Wills, BSDH, and Tori York, BSDH, for their support in performing intraoral scans during the study.
DISCLOSURE
At the time of this writing, the Oral Health System by Fresh Health Inc. is not yet available on the market. Fresh Health Inc. funded this research. Kristina Cook, MASc, is an employee of Fresh Health Inc.
ABOUT THE AUTHORS
Kimberly R. Milleman, RDH, PhD
Director, Compliance Specialist, Gold Standard Examiner and Principal Investigator, Salus Research, Inc., Fort Wayne, Indiana
Jeffery L. Milleman, DDS, MPA
Director, Clinical Operations and Principal Investigator, Salus Research, Inc., Fort Wayne, Indiana
Megan Gaff, BSDH
Dental Hygienist, Examiner, Salus Research, Inc., Fort Wayne, Indiana
Kristina Cook, MASc
Head of Clinical Engineering, Fresh Health Inc., Mountain View, California
Luis Mateo, MSc
President, Consultant, LRM Statistical Consulting, West Orange, New Jersey
References
1. Li Y, Lee S, Hujoel P, et al. Prevalence and severity of gingivitis in American adults. Am J Dent. 2010;23(1):9-13.
2. Eke PI, Thornton-Evans GO, Wei L, et al. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. 2018;149(7):576-588.e6.
3. Murakami S, Mealey BL, Mariotti A, Chapple ILC. Dental plaque-induced gingival conditions. J Periodontol. 2018;89 suppl 1:S17-S27.
4. van der Weijden F, Slot DE. Oral hygiene in the prevention of periodontal diseases: the evidence. Periodontol 2000. 2011;55(1):104-123.
5. Könönen E, Gursoy M, Gursoy UK. Periodontitis: a multifaceted disease of tooth-supporting tissues. J Clin Med. 2019; 8(8):1135.
6. Schätzle M, Löe H, Lang NP, et al. The clinical course of chronic periodontitis. J Clin Periodontol. 2004;31(12):1122-1127.
7. Arigbede AO, Babatope BO, Bamidele MK. Periodontitis and systemic diseases: a literature review. J Indian Soc Periodontol. 2012;16(4):487-491.
8. Bui FQ, Almeida-da-Silva CLC, Huynh B, et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42(1):27-35.
9. Martínez-García M, Hernández-Lemus E. Periodontal inflammation and systemic diseases: an overview. Front Physiol. 2021;12:709438.
10. Chapple ILC, Van der Weijden F, Doefer C, et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42 suppl 16:S71-S76.
11. Ramsay DS. Patient compliance with oral hygiene regimens: a behavioural self-regulation analysis with implications for technology. Int Dent J. 2000;suppl creating a successful:304-311.
12. Ng E, Lim LP. An overview of different interdental cleaning aids and their effectiveness. Dent J (Basel). 2019;7(2):56.
13. Husseini A, Slot DE, Van der Weijden GA. The efficacy of oral irrigation in addition to a toothbrush on plaque and the clinical parameters of periodontal inflammation: a systematic review. Int J Dent Hyg. 2008;6(4):304-314.
14. Rosema NAM, Hennequin-Hoenderdos NL, Berchier CE, et al. The effect of different interdental cleaning devices on gingival bleeding. J Int Acad Periodontol. 2011;13(1):2-10.
15. Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011;55(1):16-35.
16. Sälzer S, Slot DE, Van der Weijden FA, Dörfer CE. Efficacy of inter-dental mechanical plaque control in managing gingivitis - a meta-review. J Clin Periodontol. 2015;42 suppl 16:S92-S105.
17. Lobene RR, Weatherford T, Ross NM, et al. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8(1):3-6.
18. Van der Weijden GA, Timmerman MF, Nijboer A, et al. Comparison of different approaches to assess bleeding on probing as indicator of gingivitis. J Clin Periodontol. 1994;21(9):589-594.
19. Rustogi KN, Curtis JP, Volpe AR, et al. Refinement of the Modified Navy Plaque Index to increase plaque scoring efficiency in gumline and interproximal tooth areas. J Clin Dent. 1992;3(suppl C):C9-C12.
20. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267-277.
21. Mythri H, Ananda SR, Prashant GM, et al. The efficacy of antiseptic mouth rinses in comparison with dental floss in controlling interproximal gingivitis. J Int Soc Prev Community Dent. 2011;1(1):31-35.
22. Patel PV, Shruthi S, Kumar S. Clinical effect of miswak as an adjunct to tooth brushing on gingivitis. J Indian Soc Periodontol. 2012;16(1):84-88.
23. Ganavadiya R, Shekar BR, Goel P, et al. Comparison of anti-plaque efficacy between a low and high cost dentifrice: a short term randomized double-blind trial. Eur J Dent. 2014;8(3):381-388.
24. Yates RJ, Newcombe RG, Addy M. Dentine hypersensitivity: a randomized, double-blind placebo-controlled study of the efficacy of a fluoride-sensitive teeth mouthrinse. J Clin Periodontol. 2004;31(10):885-889.
25. Milleman K, Milleman J, Bosma ML, et al. Role of manual dexterity on mechanical and chemotherapeutic oral hygiene regimens. J Dent Hyg. 2022;96(3):35-45.
26. Sambunjak D, Nickerson JW, Poklepovic T, et al. Flossing for the management of periodontal diseases and dental caries in adults. Cochrane Database Syst Rev. 2011;(12):CD008829.