C. Ram Goyal, BDS; Jimmy G. Qaqish, BSc; Reinhard Schuller, MSc; and Deborah M. Lyle, RDH, BS, MS
Objective: To determine the safety of the Waterpik® Water Flosser on multiple settings as measured by clinical attachment level (CAL) and probing pocket depth (PPD) over 6 weeks. Methods and Materials: 103 subjects completed this randomized, 6-week, single-masked, three-group, parallel clinical trial. Subjects were randomly assigned to one of three treatment groups: water flosser (WF) plus a manual toothbrush, manual toothbrushing and flossing (MTF), or manual toothbrushing alone (MT). All subjects received both written and verbal instructions and demonstrated proficiency with their assigned devices at baseline. Data were evaluated at 2 weeks, 4 weeks, and 6 weeks for CAL and PPD on six teeth (Ramfjord teeth) and outcomes reported for all teeth, anterior only, and posterior only. Paired t-test was used to detect changes within groups and one-way analysis of variance (ANOVA) was used to detect differences between the groups. Results: The WF group demonstrated an improvement in CAL and PPD that exceeded those seen in the MTF and MT groups. The MT group had diminished health as measured by increases in CAL and PPD. Conclusion:The Waterpik Water Flosser is safe to use and demonstrated stability or slight improvements in periodontal health as measured by CAL and PPD at multiple pressure settings.
Also known as a dental water jet or oral irrigator, the water flosser has been on the market since 1962. Research on the water flosser spans 50 years and has evaluated the efficacy and safety on clinical signs of inflammation and dental biofilm.1-20 Studies have also examined host modulation,7,8 impact on individuals with diabetes,7 implants,6,9 and orthodontics.4,10,11 Five clinical studies have been published that showed a water flosser was significantly more effective for improving oral health than dental floss.2-6More than 70 studies demonstrate a water flosser significantly reduces bleeding, gingival inflammation, dental biofilm, probing pocket depth (PPD), pro-inflammatory mediators, subgingival bacteria, and calculus accumulation.1-25
The studies primarily are randomized controlled trials that have been published in peer-reviewed journals providing information to help clinicians make evidence-based decisions on self-care recommendations. Despite the public's use of the water flosser for decades and the sound body of evidence from the clinical studies performed to date, there still remains skepticism by some dental professionals as to the device's safety and efficacy. Previous studies have measured the impact of pulsating water on tissue both clinically and microscopically.1-25 To date no adverse effects or events have been reported. Histological findings show a reduction in inflammation,21-23 stability of PPDs and clinical attachment levels (CALs) or an improvement in these measurements,7.8,13,17removal of subgingival bacteria,14,24,25 and improvement in morphological subgingival flora.24
This article presents a randomized, controlled, parallel clinical trial designed to evaluate CALs and PPDs over 6 weeks at different pressure settings on a water flosser. Manual toothbrushing and water flossing were compared to manual toothbrushing and dental floss use and to manual toothbrushing alone. The efficacy was measured through stability or changes in both CAL and PPD. This study was approved by the All Sum Research Institutional Review Board.
Methods and Materials
Subjects
One hundred and five healthy, nonsmoking adults were randomized into one of three groups using a 1:1:1 ratio. Subjects were included in this study if they were between the ages of 25 and 70, had scoreable Ramfjord teeth (Nos. 3, 9, 12, 19, 25, and 28 using the Universal dental numbering system) (Figure 1), and had no partial dentures, orthodontic brackets, wires, or other appliances. Subjects were nonsmokers in good general health and able to provide written informed consent. They agreed to participate in the study, return for the scheduled visits, follow study protocol, and abstain from using any nonstudy devices or oral care products (Table 1). Subjects were disqualified if they had PPDs >5 mm, advanced periodontitis, or visual caries, were taking medications that can influence gingival health, or used antibiotics within 6 months of the study.
Study Design
This was a single-masked, parallel, three-group, 6-week clinical trial. Subjects were randomized into one of three groups of 35 subjects each. Group 1 (WF) received a water flosser (Waterpik® Water Flosser model 660W, Water Pik, Inc., waterpik.com) and an American Dental Association standard manual toothbrush (Oral-B® Indicator™ 35, Procter & Gamble, oralb.com). Group 2 (MTF) received the same ADA standard manual toothbrush and ADA standard dental floss (Reach® Mint flavored waxed dental floss, Johnson & Johnson Consumer, Inc., reachfloss.com), and Group 3 (MT) used the ADA standard manual toothbrush only.
All groups used the same dentifrice provided (Colgate® Cavity Protection, Colgate-Palmolive, colgate.com). Data were collected at baseline, 3 weeks, and 6 weeks. All groups received written and verbal instructions and a demonstration on the use of their assigned products. Subjects were required to demonstrate proper usage in their own mouth at the baseline visit. WF group received specific instructions and a calendar with the required pressure setting for each 2-week period (Table 2).
Oral examinations were completed and recorded at baseline, 2 weeks, 4 weeks, and 6 weeks. CALs and PPDs were assessed at six sites per study tooth and recorded at all time-points. The fixed reference point for assessing CAL levels was the cementoenamel junction. The average for CAL and PPD was recorded for each patient. One examiner collected and scored all data at all visits and was blinded to the group allocation. A UNC 15 probe was used.
All subjects brushed twice a day for a timed 2 minutes, once in the morning and once in the evening, following instructions for the Bass brushing technique. Subjects either used their own smart phone or were provided a timer to time the 2 minutes. Subjects in Group 1 used the water flosser once a day in the evening with 600 ml of warm water, and Group 2 used dental floss once a day in the evening. After brushing, the subjects rinsed with water. Subjects were required to demonstrate proper technique of their assigned product during the baseline visit. Instructions were reviewed and technique was evaluated at the 2-week visit.
Data Analysis
The primary outcome was CALs for anterior teeth, posterior teeth, and all teeth. The initial analysis compared the mean change after 6 weeks within each group using a paired t-test. A one-way analysis was used to determine differences between the groups. The secondary outcome of PPD was analyzed the same way. Data were summarized using descriptive statistics by and between treatment groups. With 35 subjects per group, the study had 90% power to detect a change in CAL of >1 mm with an effect size of 0.5 or greater. There were no changes from the planned analysis for this study.
Gingival index, bleeding on probing, and plaque index were not included in this study, as its intention was only to compare the regimens on the impact of PPD and CAL and to focus on the question of safety.
Data Management
Data were collected on case report forms (CRFs) for each subject and recorded in writing. Errors were corrected by striking a single line through the invalid data and initialed and dated by the recorder who then entered the correct data. The CRFs were completed in entirety, reviewed for completeness and accuracy, and signed by the examiner. The CRFs underwent key batch entry and verification by the statistician.
Results
One hundred and three subjects completed the study. One subject dropped out of the study for personal reasons and one subject no longer qualified at the baseline visit. There were no adverse events reported during the study. Oral examinations were negative for any oral lesions, trauma, or other abnormal findings at baseline for all subjects and at each examination visit.
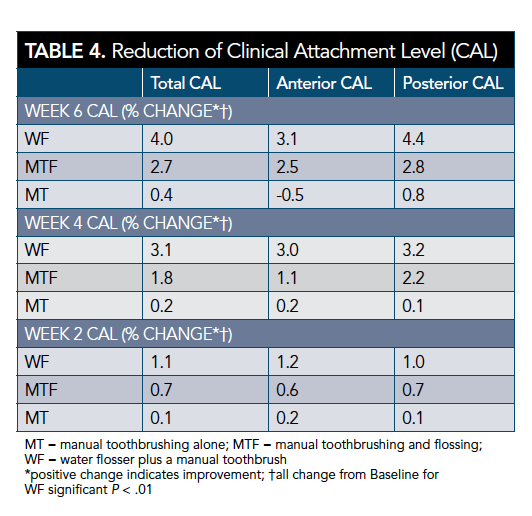
The WF group showed an improvement of CALs (4% [SD = 0.07]). The levels exceeded those of the MTF and MT groups. The MT group showed a negative change for anterior CAL (-0.5% [SD = 0.05]). These results suggest that the water flosser is at least comparable if not better than the other groups in improvement or stability of CAL (Table 3 and Table 4).
The WF group showed a reduction in PPD over the 6 weeks and exceeded the reductions seen for the MTF and MT groups. The MT group showed a slight increase for anterior PPD. The results demonstrate that the water flosser is at least comparable if not better than MTF and MT in the reduction or stability of PPD over 6 weeks (Table 3 and Table 5).
Discussion
Numerous clinical studies have evaluated the safety of the Waterpik Water Flosser on oral soft and hard tissues. To date there have been no adverse events reported in randomized controlled trials. Anecdotally, some dental professionals feel that the pressure must be dangerous to the epithelial attachment or push bacteria deeper into the pocket. This has not been shown in any of the studies conducted using the Waterpik Water Flosser since its introduction in 1962.1-25 Those studies used a similar range of pressure as was used in this study, primarily from 50 psi to 100 psi, mostly using the classic water flosser tip.
To the contrary, studies have reported data that refutes these assumptions. Microscopic tissue samples from water-flossed sites have shown a reduction in inflammation in pockets up to 6 mm.21-23 In contrast the control group showed diffuse moderate inflammation and connective tissue disorganization, fewer inflammatory cells, and vascular congestion.
An ultrastructural study evaluated the impact of water flossing on the epithelial wall and the morphologic types of bacteria in subjects with periodontally involved teeth that were scheduled for extraction. Scanning electron microscopy showed that the water-flossed sites showed considerable reduction in the number of microorganisms in sites from 0 to 6 mm.24 Studies have also shown a reduction in spirochetes25and Prevotella intermedia in sites 4 mm to 6 mm.14 Adolescent subjects with fixed orthodontic appliances who water flossed had an 80% better reduction in total aerobic flora and a 60% better reduction in lactobacillus counts when compared with control group.10 Studies have also shown a reduction in pro-inflammatory mediators measured in the gingival crevicular fluid or serum.7,8
The present study was designed to evaluate the safety of water flossing on multiple pressure settings as measured by the CAL (primary objective) and PPD (secondary objective). The data showed that the water-flosser group had similar or better changes than string floss or manual toothbrushing alone. The same was reported for PPD.
The outcomes from this study support previously published data on CAL and PPD. A study on subjects with diabetes showed improvements in CAL or PPD for the water flosser group and the control group at 3 months.7 There were no differences between the groups. In a 2-week study, reductions in PPD were significantly better than control group in mild-to-moderate periodontitis subjects.8 Two 6-month studies with subjects in periodontal maintenance programs showed a statistically significant improvement in PPD within the group that used the water flosser.13,17
Conclusion
The Waterpik Water Flosser is safe to use and demonstrated stability in CALs and PPDs. The results compared favorably to manual toothbrushing and dental floss or manual toothbrushing alone. This study should alleviate concerns that the Waterpik Water Flosser, regardless of pressure, is associated with a negative impact on gingival tissue or epithelial attachments as measured by CAL and PPD.
About the Authors
C. Ram Goyal, BDS
President and Principle Investigator, All Sum Research Center Ltd.,
Mississauga, Ontario, Canada
Jimmy G. Qaqish, BSc
Vice President of Clinical Operations, All Sum Research Center Ltd., Mississauga, Ontario, Canada
Reinhard Schuller, MSc
Senior Consultant, Reinhard Schuller Consulting,
Toronto, Ontario, Canada
Deborah M. Lyle, RDH, BS, MS
Director of Professional and Clinical Affairs, Water Pik, Inc.,
Fort Collins, Colorado
References
1. Lobene RR. The effect of a pulsed water pressure cleansing device on oral health. J Periodontol. 1969;40(11):667-670.
2. Barnes CM, Russell CM, Reinhardt RA, et al. Comparison of irrigation to floss as an adjunct to tooth brushing: effect on bleeding, gingivitis, and supragingival plaque. J Clin Dent. 2005;16(3):71-77.
3. Rosema NA, Hennequin-Hoenderdos NL, Berchier CE, et al. The effect of different interdental cleaning devices on gingival bleeding. J Int Acad Periodontol. 2011;13(1):2-10.
4. Sharma NC, Lyle DM, Qaqish JG, et al. Effect of a dental water jet with orthodontic tip on plaque and bleeding in adolescent patients with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2008;133(4):565-571.
5. Goyal CR, Lyle, DM, Qaqish JG, Schuller R. Evaluation of the plaque removal efficacy of a water flosser compared to string floss in adults after a single use. J Clin Dent. 2013;24(2):37-42.
6. Magnuson B, Harsono M, Stark PC, et al. Comparison of the effect of two interdental cleaning devices around implants on the reduction of bleeding: a 30-day randomized clinical trial. Compend Contin Educ Dent. 2013;34(spec iss 8):2-7.
7. Al-Mubarak S, Ciancio S, Aljada A, et al. Comparative evaluation of ad-junc-tive oral irrigation in diabetics. J Clin Periodontol. 2002;29(4):295-300.
8. Cutler CW, Stanford TW, Abraham C, et al. Clinical benefits of oral irrigation for periodontitis are related to reduction of pro-inflammatory cytokine levels and plaque. J Clin Periodontol. 2000;27(2):134-143.
9. Felo A, Shibly O, Ciancio SG, et al. Effects of subgingival chlorhexidine irrigation on peri-implant maintenance. Am J Dent. 1997;10(2):107-110.
10. Hurst JE, Madonia JV. The effect of an oral irrigating device on the oral hygiene of orthodontic patients. J Am Dent Assoc. 1970;81(9):678-682.
11. Burch JG, Lanese R, Ngan P. A two-month study of the effects of oral irrigation and automatic toothbrush use in an adult orthodontic population with fixed appliances. Am J Orthod Dentofacial Orthop. 1994;106(2):121-126.
12. Goyal CR, Lyle DM, Qaqish JG, Schuller R. The addition of a water flosser to power tooth brushing: effect on bleeding, gingivitis, and plaque. J Clin Dent. 2012;23(2):57-63.
13. Newman MG, Flemmig TF, Nachnani S, et al. Irrigation with 0.06% chlorhexidine in naturally occurring gingivitis. II. 6 months microbiological observations. J Periodontol. 1990;61(7):427-433.
14. Chaves ES, Kornman KS, Manwell MA, et al. Mechanism of irrigation effects on gingivitis. J Periodontol. 1994;65(11):1016-1021.
15. Ciancio SG, Mather ML, Zambon JJ, Reynolds HS. Effect of a chemotherapeutic agent delivered by an oral irrigation device on plaque, gingivitis, and subgingival microflora. J Periodontol. 1989;60(6):310-315.
16. Newman MG, Cattabriga M, Etienne D, et al. Effectiveness of adjunctive irrigation in early periodontitis: multi-center evaluation. J Periodontol. 1994;65(3):224-229.
17. Flemmig TF, Epp B, Funkenhauser Z, et al. Adjunctive supragingival irrigation with acetylsalicylic acid in periodontal supportive therapy. J Clin Periodontol. 1995;22(6):427-433.
18. Flemmig TF, Newman MG, Doherty FM, et al. Supragingival irrigation with 0.06% chlorhexidine in naturally occurring gingivitis. I. 6 month clinical observations. J Periodontol. 1990;61(2):112-117.
19. Gorur A, Lyle DM, Schaudinn C, Costerton JW. Biofilm removal with a dental water jet. Compend Contin Educ Dent. 2009;30(spec iss 1):1-6.
20. Goyal CR, Lyle DM, Qaqish JG, Schuller R. Efficacy of two interdental cleaning devices on clinical signs of inflammation: a four-week randomized controlled trial. J Clin Dent. 2015; 26(2):55-60.
21. Lainson PA, Bergquist JJ, Fraleigh CM. A longitudinal study of pulsating water pressure cleansing devices. J Periodontol. 1972;43 (7):444-446.
22. Krajewski JJ, Giblin J, Gargiulo AW. Evaluation of a water pressure cleansing device as an adjunct to periodontal treatment. Periodontics. 1964;2(2):76-78.
23. Canter MT, Stahl SS. Interdental col tissue responses to the use of a water pressure cleansing device. J Periodontol. 1969;40(5):292-295.
24. Cobb CM, Rodgers RL, Killoy WJ. Ultrastructural examination of human periodontal pockets following the use of an oral irrigation device in vivo. J Periodontol. 1988;59(3):155-163.
25. Drisko CL, White CL, Killoy WJ, Mayberry WE. Comparison of dark-field microscopy and a flagella stain for monitoring the effect of a Water Pik on bacterial motility. J Periodontol. 1987;58(6):381-386