C. Ram Goyal, BDS; Jimmy G. Qaqish, BSc; Reinhard Schuller, MSc; and Deborah M. Lyle, RDH, BS, MS
Objective: To evaluate the effectiveness of a novel sonic toothbrush, compared with a traditional sonic toothbrush and manual brushing and flossing, on gingival inflammation and plaque scores. Methods and Materials: 105 subjects completed this randomized, 4-week, single-masked, three-group, parallel clinical trial. Subjects were randomly assigned to one of three treatment groups: novel sonic toothbrush, traditional sonic toothbrush, or manual toothbrushing and flossing (MTF). All subjects received both written and verbal instructions and demonstrated proficiency with their assigned devices at baseline. Data were evaluated at baseline, 2 weeks, and 4 weeks for bleeding on probing (BOP), gingival inflammation (MGI), and Rustogi Modified Navy Plaque Index. Results: All groups demonstrated a significant reduction in BOP and MGI at 2 weeks and 4 weeks (P < .001). The novel sonic toothbrush was significantly more effective than the traditional sonic toothbrush and MTF for BOP and MGI at week 4. All regimens demonstrated a significant reduction in plaque accumulation at 2 weeks and 4 weeks except the traditional sonic toothbrush marginal (P = .80) and MTF marginal (P = .53) at 2 weeks. The novel sonic toothbrush was more effective than the traditional sonic toothbrush and MTF for whole-mouth plaque reduction at 4 weeks. Conclusion:The novel sonic toothbrush was significantly more effective than a traditional sonic toothbrush or standard brushing and flossing for improving oral health over 4 weeks.
Effective daily plaque control is the foundation to prevent and control oral infections.1 Globally, the most widely used device to control supragingival plaque is a toothbrush.2 Evidence shows that toothbrushing can reliably control plaque, provided the technique is thorough and performed at a high standard on a daily basis. Establishing and maintaining effective oral hygiene requires patient motivation, appropriate devices, and repeated professional oral hygiene instruction.3
A meta-analysis of single-use studies showed that use of a manual toothbrush leads to plaque reductions of approximately 42% from baseline scores.4 There are no data from the meta-analysis for the reduction of gingival inflammation; however, individual studies have shown a reduction of inflammation, though the effect estimate is currently unknown.4 Bristle designs vary greatly from brush to brush; reductions based on design have been reported at 24% to 47% for flat-trim bristles, 33% to 54% for multilevel bristles, and 39% to 61% for crisscross bristles.1,4 Specific differences between the designs were not available, nor was information regarding the reduction of gingival inflammation.
Power toothbrushing has shown a 46% reduction from baseline in plaque indices after a one-time brushing.1,4 Rechargeable power brushes have proven to be more effective than replaceable battery devices, and oscillating-rotating brushes have a slight statistical advantage over side-to-side motion, but the clinical significance is questionable. Comparison of manual to power toothbrushing shows 11% to 21% better reduction in plaque scores and 6% to 11% better reduction in gingival inflammation scores.1,4
The weakness in the data is the lack of information regarding gingival health. Demonstrating a reduction in plaque does not automatically demonstrate a concurrent reduction in gingival inflammation. The plaque indices used address only the supragingival plaque, but the primary culprit of gingival inflammation is the subgingival plaque, especially in the interdental areas. Thus, it may be more important to measure gingival health as the primary outcome of effectiveness.
The purpose of this randomized, controlled trial was to evaluate the effectiveness of a novel sonic toothbrush, compared with a traditional sonic toothbrush or manual brushing and flossing, on gingival inflammation and plaque scores.
Methods and Materials
The study was an examiner-masked, three-treatment, parallel-group, randomized, controlled clinical trial. It was designed to evaluate the reduction in inflammation and plaque from a novel sonic toothbrush (Waterpik® Sonic-Fusion [WSF], Water Pik, Inc., waterpik.com), compared with a traditional sonic toothbrush (ST) and manual toothbrushing and flossing (MTF). One hundred and five subjects who met the inclusion criteria were enrolled in the study (Table 1 and Table 2). Subjects were allocated to one of three groups based on a 1:1:1 randomization sequence. Group 1 used the novel sonic toothbrush, WSF; Group 2 used the traditional sonic toothbrush, ST ; and Group 3 used a manual toothbrush (Oral-B® Indicator™ 35, Procter & Gamble, oralb.com) and waxed, unflavored dental floss (Reach® dental floss, Johnson & Johnson Consumer, Inc., reachfloss.com). All subjects used a standard American Dental Association (ADA) toothpaste (Colgate® Cavity Protection toothpaste, Colgate-Palmolive, colgate.com). The study end point was the 4-week scores for bleeding on probing (BOP), modified gingival index (MGI), and Rustogi Modification of the Navy Plaque Index (RMNPI).
Subjects abstained from brushing for 12 to 14 hours before baseline, 2-week, and 4-week data collection. They then used their assigned dental devices twice a day, once in the morning and once in the evening. (Though subjects did not complete a diary to document compliance, they agreed to follow instructions when they signed the consent form.) One examiner collected and scored all subjects and was masked to the product allocation. A UNC 15 probe was used, and the examiner rounded up or down depending on which marking was closest; if in the middle, the examiner rounded up. Oral soft and hard tissue was evaluated at baseline, 2 weeks, and 4 weeks. BOP was recorded for six sites per tooth and scored on a binary scale of 0 for no bleeding or 1 for bleeding. Bleeding within 30 seconds was considered positive. MGI was scored on all teeth and for whole-mouth, facial, and lingual surfaces, using a 0 to 4 scale (Figure 1).5
Subjects rinsed for 1 minute with erythrosine disclosing solution (FD&C #3, Germiphene Corp., germiphene.com) and then rinsed with water before scoring the RMNPI. The RMNPI divides the tooth into nine sections and emphasizes the marginal and approximal regions (Figure 2).6
During the baseline visit, subjects received their allocated products. The traditional sonic toothbrush had the ramp-up control deactivated before it was dispensed to the group. Instructions were provided by demonstration and verbally, and the subjects demonstrated proper use of the devices. Written instructions were provided for reference at home. Each subject was told to use the assigned device(s) once in the morning and once in the evening. All subjects brushed for 2 minutes. WSF group used the water flosser mode for 1 minute.
Statistical Analysis and Management
The primary objective of this randomized trial was to determine the percentage of BOP sites after 4 weeks. One-way analysis of variance (ANOVA) was used to determine the mean change among the three groups. The arcsine transformation was used to stabilize the variances of the percentage data.7 The transformed data were used in the analysis. However, the tables in this report present the mean of observed subject-specific percentages for the groups. The analysis method was also used to determine the outcomes for BOP at 2 weeks and MGI at 2 and 4 weeks.
The secondary objective for the assessment of change in plaque accumulation used ANOVA for 2-week and 4-week change from baseline. Results are reported for whole-mouth, proximal, and marginal regions, and for facial and lingual surfaces. Oral soft-tissue examination findings were documented at baseline, 2 weeks, and 4 weeks.
Data were collected on case report forms (CRFs) for each subject and recorded in writing. Errors were corrected by striking a single line through invalid data and initialed and dated by the recorder, who then entered the correct data. The CRFs were completed in entirety, reviewed for completeness and accuracy, and signed by the examiner. The CRFs underwent key batch entry and verification. Data were tabulated according to clinical scoring appropriate for the assessment instrument used. Data were summarized with descriptive statistics (mean, minimum, maximum, and standard deviation) for each treatment group and overall. Post study power calculations determined the study had in excess of 90% power to detect meaningful differences in clinical signs of inflammation with 35 subjects per group.
Results
All 105 subjects completed the study. The gender breakdown was 84 female subjects and 21 male subjects. The mean age was 50.1 years, with the minimum age being 25 years and the maximum being 68 years. Both age (P = .104) and gender (P = .273) were comparable among the treatment groups at baseline. All subjects were nonsmokers (Table 1).
There were no differences between treatment groups at baseline for gingival and bleeding scores (P > .073) and plaque scores (P > .372). No adverse events were reported during the study for any subject in any group. Oral soft-tissue examinations at baseline, 2 weeks, and 4 weeks indicated normal findings.
Gingival Inflammation
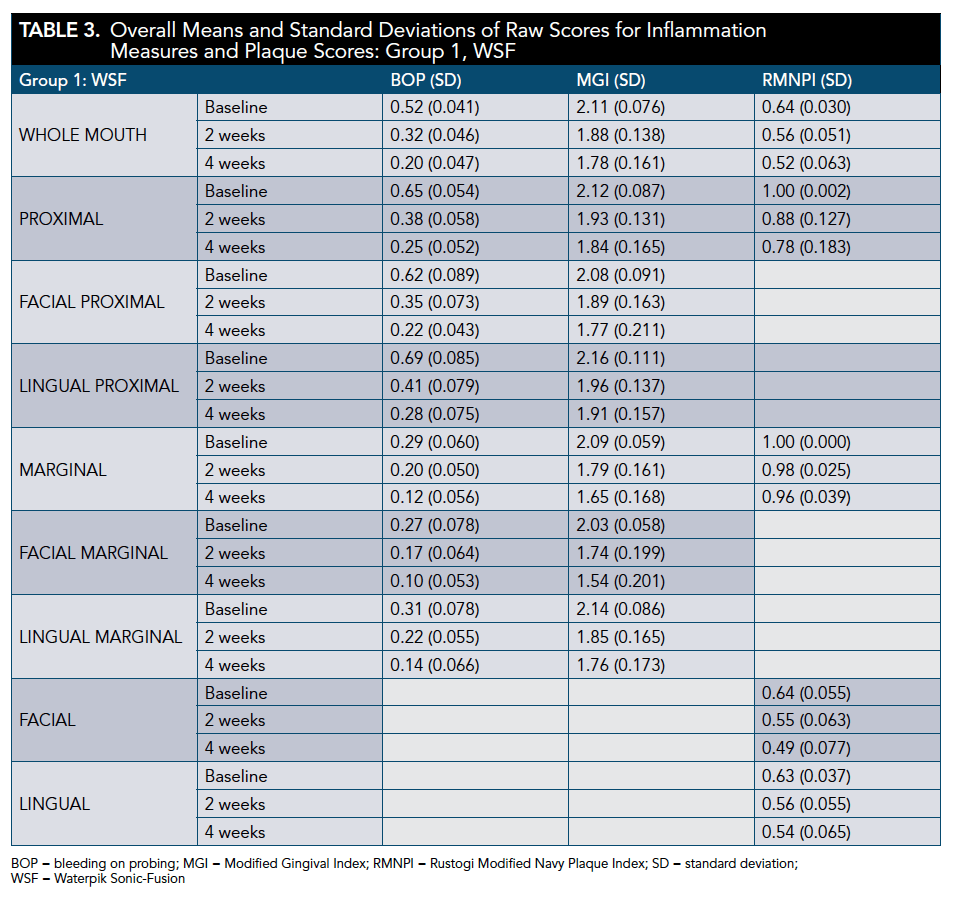
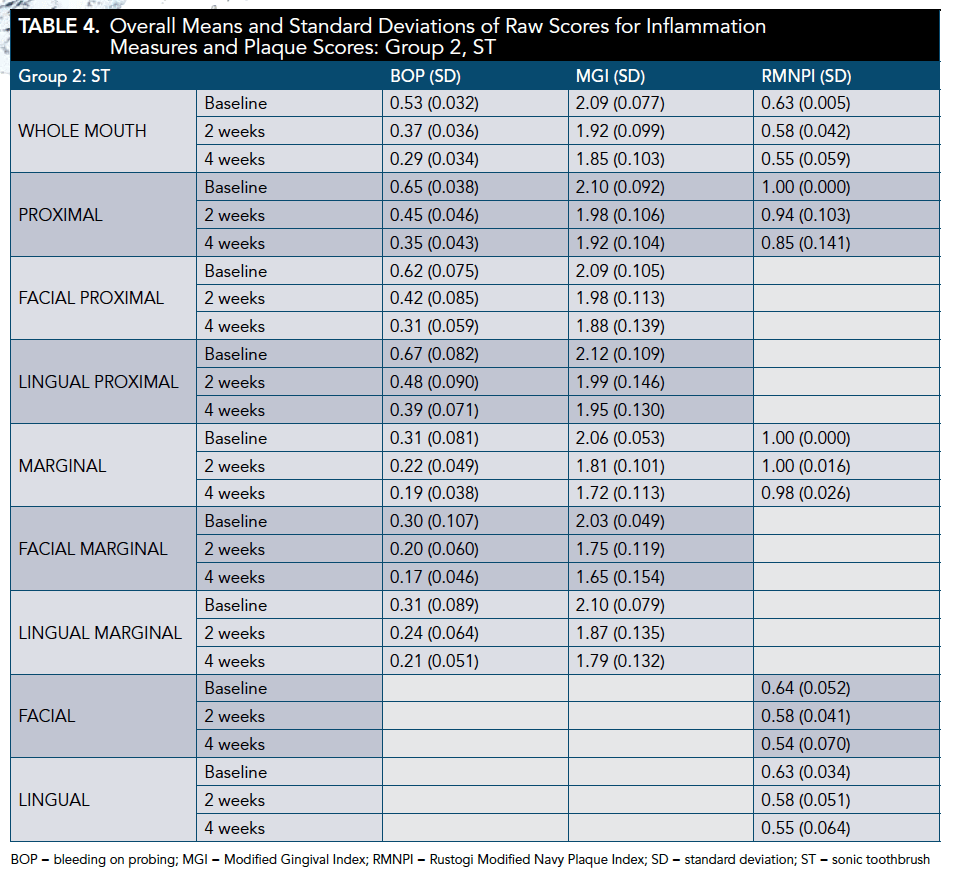
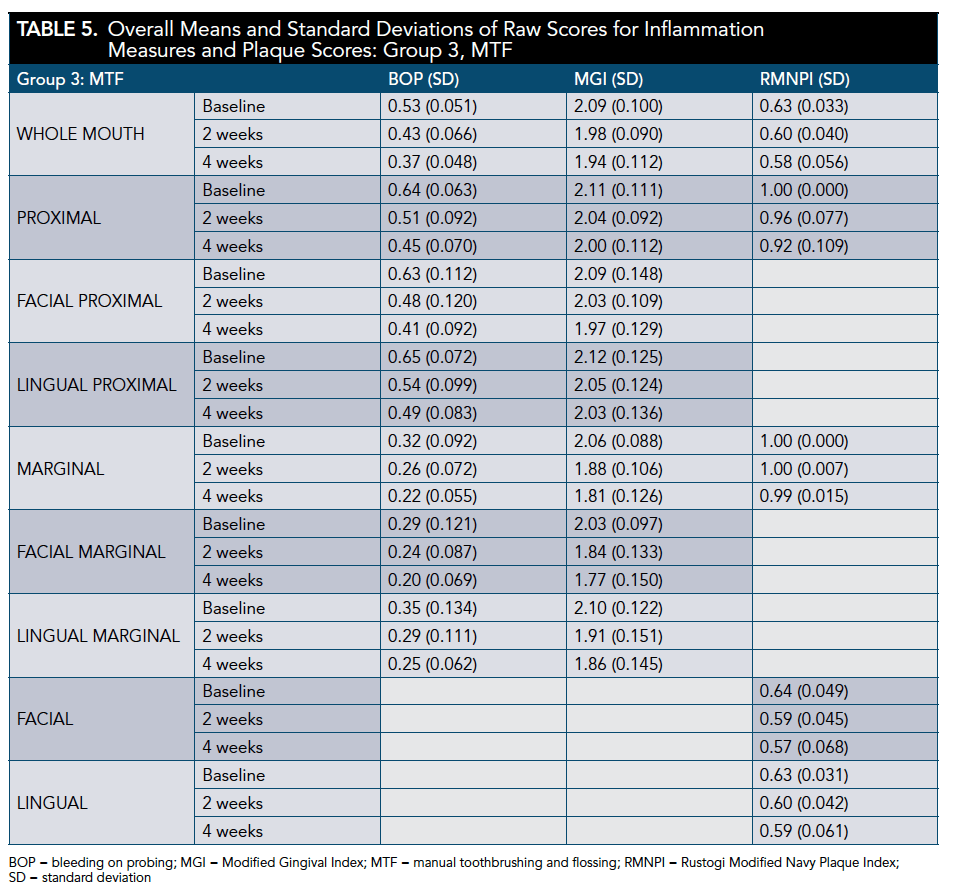
All groups demonstrated a significant reduction in BOP and MGI at 2 and 4 weeks (P <.001). The WSF was significantly more effective than the ST for BOP whole-mouth, proximal, and gingival margin at 4 weeks (P < .001). The WSF was also significantly more effective than the MTF regimen for all BOP areas measured at 4 weeks (P < .001) (Table 3 through Table 6).
The WSF was significantly more effective than the ST for whole-mouth (P < .001), proximal (P < .001), and marginal (P = .008) MGI scores at 4 weeks. Similarly, the WSF was significantly more effective than the MTF for all areas measured (P < .001) (Table 3 through Table 5 and Table 7).
Plaque Data
All regimens demonstrated a significant reduction in plaque accumulation at 2 weeks (P < .001; except ST marginal [P = .080], ST proximal [P = .002], MTF marginal [P = .053], and MTF proximal [P = .008]) and 4 weeks (P < .001; except MTF marginal [P = .002]). The WSF was significantly more effective than the ST for whole-mouth plaque and more effective than the MTF for all areas measured at 4 weeks (Table 3 through Table 5 and Table 8).
Discussion
Many powered toothbrushes are on the market. Technological advances have taken toothbrushes to a new level, providing unique ways to improve oral health. Some devices include smart technology that helps individuals brush for 2 minutes, control pressure against the tooth and gingival tissue, and see where they are neglecting to brush.8,9 Additionally, brush heads are designed to provide greater access to line angles and slightly below the gingival margin. Regardless of brushing motion, brush-head design, or smart technology, the cleaning of inaccessible or difficult-to-access areas in the mouth may benefit from a different device.
The use of a sonic toothbrush and a water flosser has been studied. Barnes and colleagues showed that sonic toothbrushing and water flossing combined were 65% more effective at reducing gingival bleeding and 92% more effective at reducing dental plaque than manual toothbrushing and flossing on facial surfaces.10 A subsequent study compared sonic brushing and water flossing to sonic toothbrushing alone and manual toothbrushing alone. The addition of a water flosser to sonic toothbrushing (Waterpik® Complete Care, Water Pik, Inc.) was 70% more effective at reducing BOP, 48% more effective for reducing gingival inflammation, and 52% more effective for removing dental plaque than a leading sonic toothbrush at 4 weeks.11
The novel sonic toothbrush in this study was designed to clean both the supra- and subgingival areas and was compared with a traditional sonic toothbrush and manual toothbrushing and flossing. The sonic action of the toothbrush is a back-and-forth motion that is designed to clean the tooth surfaces, line angles, and supragingival areas. With the click of a button, the brush head becomes a jet tip for water flossing. This hydrodynamic action, which is the same water-flossing action used in all other Waterpik models, allows pulsating water to efficiently and effectively remove plaque and debris from the interdental space, proximal surface of the teeth, and subgingivally, with concomitant reduction in gingival inflammation and bleeding.10-26 The dual action allows a thorough oral-hygiene routine with a single device.
Patients desire easy-to-use devices that have demonstrated effectiveness. It is well-documented that people do not like to floss, do not floss correctly, and will readily choose another device over floss.27-29 The Sonic-Fusion was superior to manual toothbrushing and flossing for all areas measured in this study, most importantly the reduction of gingival bleeding and inflammation. The ADA Seal of Acceptance Program states that the only accepted products that will be allowed to make plaque control claims will be those that can also demonstrate a significant effect against gingivitis. If a product can only demonstrate a significant plaque reduction without a concomitant significant reduction in gingivitis, it will not be eligible for Acceptance.30
Outcomes for the Waterpik Water Flosser show statistically significant reductions in gingival inflammation, gingival bleeding, and plaque removal compared with toothbrushing and flossing, interdental brushes, and an air flosser.10,15-17 It has also showed significant benefits for individuals with fixed orthodontic appliances, gingivitis, implants, or type 2 diabetes, or in a periodontal maintenance program.10,11,16-24
This study provided data on the efficacy of a novel sonic toothbrush, WSF. It demonstrated superiority to the ST and to traditional brushing and flossing.
Conclusion
The results from this study demonstrated the following: (1) The WSF is twice as effective as manual toothbrushing and flossing for reducing gingival bleeding and inflammation overall and interdentally. (2) The WSF is significantly more effective than ST for reducing gingival bleeding and inflammation overall and interdentally. (3) The WSF is significantly more effective than manual toothbrushing and ST for reducing overall plaque scores. Though the WSF is a novel sonic toothbrush, its water-flossing action, which is the key to its efficacy, is long established. The device enables sonic toothbrushing and water flossing to be accomplished in a single step.
About the Authors
C. Ram Goyal, BDS
President and Principle Investigator, All Sum Research Center Ltd.,
Mississauga, Ontario, Canada
Jimmy G. Qaqish, BSc
Vice President of Clinical Operations, All Sum Research Center Ltd.,
Mississauga, Ontario, Canada
Reinhard Schuller, MSc
Senior Consultant, Reinhard Schuller
Consulting,
Toronto, Ontario, Canada
Deborah M. Lyle, RDH, BS, MS
Director of Professional and Clinical Affairs, Water Pik, Inc.,
Fort Collins, Colorado
References
1. Chapple ILC, Van der Weijden F, Doerfer C, et al. Primary prevention of periodontitis: managing gingivitis. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42(suppl 16):S71-S76.
2. Van Der Weijden F, Slot DE. Oral hygiene in the prevention of periodontal disease: the evidence. Periodontol 2000. 2011;55(1):104-123.
3. Tonetti MS, Eickholz P, Loos BG, et al. Principles in prevention of periodontal diseases. Consensus report of group 1 of the 11th European workshop on periodontology on effective prevention of periodontal diseases and peri-implant diseases. J Clin Periodontol. 2015;42(suppl 16):S5-S11.
4. Van der Weijden FA, Slot DE. Efficacy of homecare regimens for mechanical plaque removal in managing gingivitis: a meta review. J Clin Periodontol. 2015;42(suppl 16):S77-S91.
5. Lobene RR, Weatherford T, Ross NM, et al. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8(1):3-6.
6. Rustogi KN, Curtis JP, Volpe AR, et al. Refinement of the Modified Navy Plaque Index to increase plaque scoring efficiency in gumline and interproximal tooth areas. J Clin Dent. 1992;3(suppl C):C9-C12.
7. Freeman MF, Kukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21(4):607-611.
8. Electric toothbrushes with Bluetooth connectivity. Oral-B. https://oralb.com/en-us/product- collections/bluetooth. Accessed May 2, 2018.
9. Electric toothbrushes. Philips Sonicare. https://www.usa.philips.com/c-m-pe/electric-toothbrushes/diamondclean-smart/latest#filters=DIAMONDCLEAN_SMART _SU&sliders=&support=&price=&priceBoxes=&page=&layout=12.subcategory.p-grid-icon. Accessed May 2, 2018.
10. Barnes CM, Russell CM, Reinhardt R, et al. Comparison of irrigation to floss as an adjunct to tooth brushing: effect on bleeding, gingivitis, and supragingival plaque. J Clin Dent. 2005;16(3):71-77.
11. Goyal CR, Lyle DM, Qaqish JG, Schuller R. The addition of a water flosser to power tooth brushing: effect on bleeding, gingivitis, and plaque. J Clin Dent. 2012;23(2):57-63.
12. Cobb CM, Rodgers RL, Killoy WJ. Ultrastructural examination of human periodontal pockets following the use of an oral irrigation device in vivo. J Periodontol. 1988;59(3):155-163.
13. Drisko CL, White CL, Killoy WJ, Mayberry WE. Comparison of dark-field microscopy and a flagella stain for monitoring the effect of a Water Pik on bacterial motility. J Periodontol. 1987;58(6):381-386.
14. Goyal CR, Lyle, DM, Qaqish JG, Schuller R. Evaluation of the plaque removal efficacy of a water flosser compared to string floss in adults after a single use. J Clin Dent. 2013;24(2):37-42.
15. Rosema NA, Hennequin-Hoenderdos, NL, Berchier CE, et al. The effect of different interdental cleaning devices on gingival bleeding. J Int Acad Periodontol. 2011;13(1):2-10.
16. Sharma NC, Lyle DM, Qaqish JG, et al. Effect of a dental water jet with orthodontic tip on plaque and bleeding in adolescent patients with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2008;133(4):565-571.
17. Magnuson B, Harsono M, Stark PC, et al. Comparison of the effect of two interdental cleaning devices around implants on the reduction of bleeding: a 30-day randomized clinical trial. Compend Contin Educ Dent. 2013;34(spec iss 8):2-7.
18. Al-Mubarak S, Ciancio S, Aljada A, et al. Comparative evaluation of adjunctive oral irrigation in diabetics. J Clin Periodontol. 2002;29(4):295-300.
19. Cutler CW, Stanford TW, Abraham C, et al. Clinical benefits of oral irrigation for periodontitis are related to reduction of pro-inflammatory cytokine levels and plaque. J Clin Periodontol. 2000;27(2):134-143.
20. Newman MG, Flemmig TF, Nachnani S et al. Irrigation with 0.06% chlorhexidine in naturally occurring gingivitis. II. 6 months microbiological observations. J Periodontol. 1990;61(7):427-433.
21. Chaves ES, Kornman KS, Manwell MA, et al. Mechanism of irrigation effects on gingivitis. J Periodontol. 1994;65(11):1016-1021.
22. Newman MG, Cattabriga M, Etienne D, et al. Effectiveness of adjunctive irrigation in early periodontitis: Multi-center evaluation. J Periodontol. 1994;65(3):224-229.
23. Flemmig TF, Epp B, Funkenhauser Z, et al. Adjunctive supragingival irrigation with acetylsalicylic acid in periodontal supportive therapy. J Clin Periodontol. 1995;22(6):427-433.
24. Flemmig TF, Newman MG, Doherty FM, et al. Supragingival irrigation with 0.06% chlorhexidine in naturally occurring gingivitis. I. 6 month clinical observations. J Periodontol. 1990;61(2):112-117.
25. Gorur A, Lyle DM, Schaudinn C, Costerton JW. The dental water jet: a product ahead of its time. Compend Contin Educ Dent. 2009;30(spec iss 1):1-6.
26. Goyal CR, Lyle DM, Qaqish JG, Schuller R. Efficacy of two interdental cleaning devices on clinical signs of inflammation: a four-week randomized controlled trial. J Clin Dent. 2015;26(2):55-60.
27. Lang WP, Farghaly MM, Ronis DL. The relations of preventive dental behaviors to periodontal health status. J Clin Periodontol. 1994;21(3):194-198.
28. Tedesco L, Keffer MA, Fleck-Kandath C. Self-efficacy, reasoned action, and oral health behavior reports: a social cognitive approach to compliance. J Behav Med. 1991;14(4):341-355.
29. Kleber CJ, Putt MS. Formation of a flossing habit using a floss-holding device. J Dent Hyg. 1990;64(3):140-143.
30. American Dental Association Council on Scientific Affairs. Acceptance Program Requirements: Toothbrushes. April 2016.