Richard Zimmermann, DDS; and Stefanie Seitz, DDS
Abstract
As implant therapy evolves, patients are increasingly desiring natural-looking, metal-free restorations. The ivory coloring of the Straumann PURE Ceramic Implant accommodates this trend, offering patients a highly esthetic outcome. In the case presented, this monotype-style implant was used along with a virtual planning workflow system that enabled real-time communication between the implant planning software and the laboratory software. The result was an appealing provisional restoration for a 30-year-old female patient.
The natural-looking ivory color of the Straumann® PURE Ceramic Implant (Straumann, www.straumann.us) not only makes the implant appear more like a real tooth but it also supports clinicians in cases of thin-gingiva biotype or soft-tissue recession. The implant’s monotype design is based on features of the Straumann® Soft Tissue Level Standard Plus and Straumann® Bone Level implants. (Author’s note: Product information has been provided by the manufacturer.)
Initial Situation
A 30-year-old woman with a non-contributory medical history presented to the University of Texas Health Science Center San Antonio School of Dentistry clinic for evaluation of a maxillary edentulous site. Review of her dental history revealed that tooth No. 12 was lost due to failed endodontic therapy approximately 1 year prior during her pregnancy. She was now ready to have the tooth replaced.
She presented with a high smile line, medium-scalloped gingiva with medium thickness, and a desire to not have any metal in her oral cavity. When discussing various options regarding implant therapy, the patient was interested in being evaluated for an all-ceramic implant.
This is a monotype-style implant, meaning the abutment and implant body are one piece. Though new to the United States, in European case documentation the implant has shown excellent osseointegration and soft-tissue response.1,2
Treatment Plan
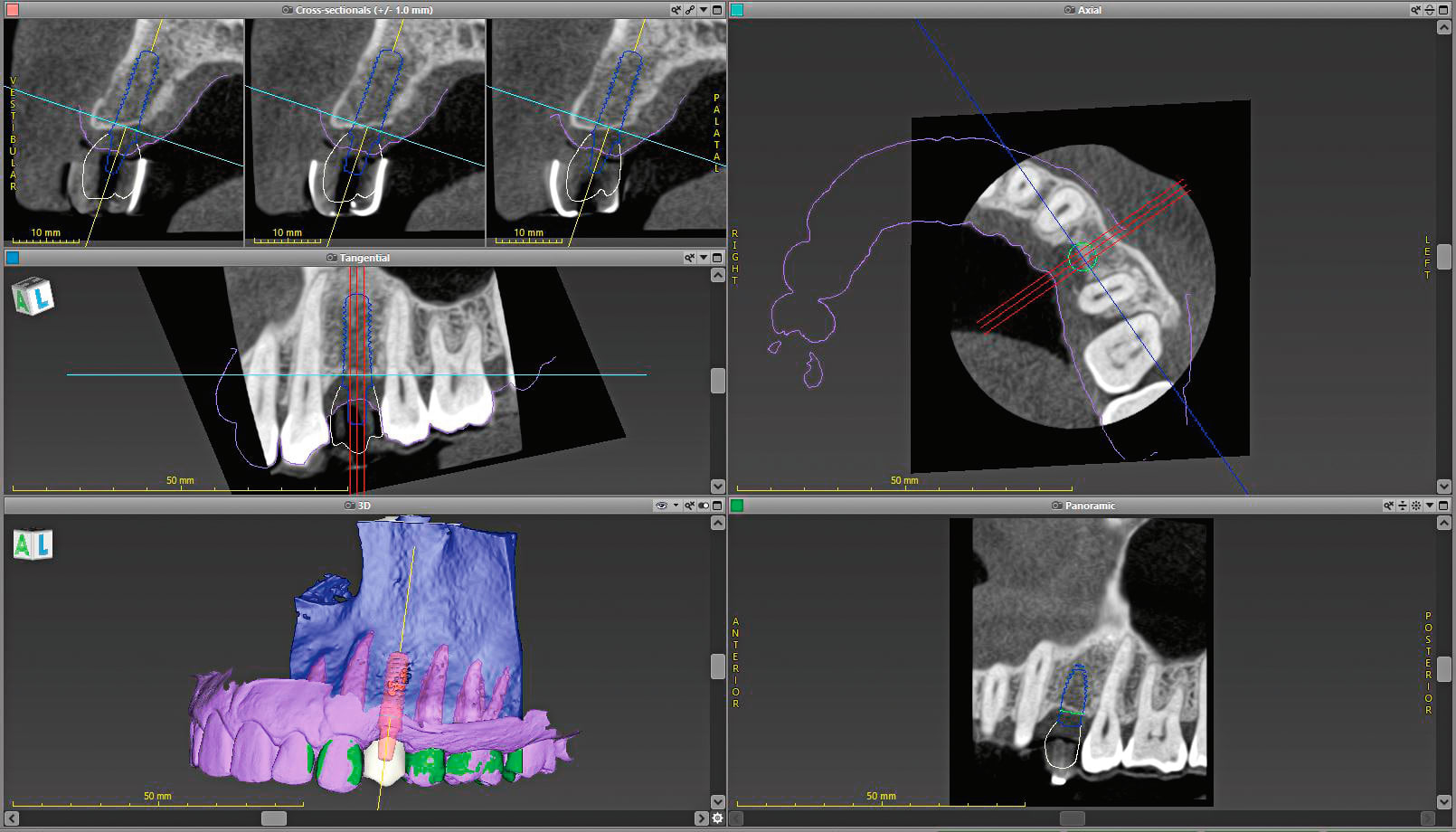
The patient was sent to have a cone-beam computed tomography (Morita, www.morita.com) taken of the area in question. Digital diagnostic impressions were also taken using an intraoral scanner (Trios® 3, 3Shape, www.3shape.com). Once these were obtained, the digital imaging and communications in medicine (DICOM) files were imported into the implant planning software (coDiagnostiX®, Dental Wings, www.dentalwings.com), while the scan files were imported into the laboratory software (Straumann® CARES® Visual, Straumann) (Figure 1 and Figure 2). Because the Straumann PURE Ceramic Implant is monobody in design and it is not recommended to modify the abutment,3 the DWOS Synergy™ workflow (Dental Wings) was utilized to virtually plan this case.
DWOS Synergy provides real-time communication between the implant planning software (coDiagnostiX) and the laboratory software (Straumann CARES Visual). This feature improves implant planning by allowing the visualization of the relationship between the proposed implant position and the proposed restoration. Modifications made to the implant position or restoration design are immediately transferred to the other software, providing instantaneous feedback on how the modification of one affects the other. Of particular interest regarding the Straumann PURE Ceramic Implant is that one can design the restoration and be assured that the planned position will not require modification for restorative materials.
Once the planning was complete, both the surgical guide and the provisional designs were sent off for fabrication; the guide was sent to a laboratory to be printed by an Objet30 OrthoDesk (Stratasys, www.stratasys.com), while the provisional file was sent to the Straumann milling center in Arlington, Texas, to be fabricated out of polycon® ae (Straumann) polymethyl methacrylate-based acrylate resin (Figure 3 and Figure 4). During the surgical planning using the DWOS Synergy workflow, a Straumann PURE Ceramic Implant (4.1 mm x 12 mm) was selected with an abutment height of 5.5 mm.
Surgical Procedure
The Straumann PURE Ceramic Implant design is a combination of the tissue-level and bone-level implants—the neck of the implant mirrors the Straumann® Tissue Level implant, while the implant body mimics the Straumann® Bone Level design (Figure 5). As such, the surgical protocol for preparing the osteotomy for the Straumann PURE Ceramic Implant is the same as the corresponding bone-level implant. For this case, a guide was used to prepare the osteotomy following the protocol set forth for bone-level implants given by coDiagnostiX. Though this case was performed with the Straumann® Guided Surgery (SGS) concept,4 a small flap was made to ensure the desired position of the Straumann PURE Ceramic Implant shoulder. SGS uses different combinations of sleeve positions, drill lengths, and drill handles to prepare the osteotomy to the correct depth. Sleeves can be placed at three different heights from the implant level (2 mm, 4 mm, or 6 mm) based on the case and the surgeon’s preference. The combination of drill length (short, long, or extra-long) and drill handle (1 mm or 3 mm) is determined by the implant planning software, which provides the surgical protocol to use at the time of surgery.
The Straumann PURE Ceramic Implant system uses a series of “position indicators” that aid in ensuring the correct position of the implant during surgery. Both abutment diameters and heights have corresponding position indicators that are placed into the osteotomy for evaluation (Figure 6). Typically, once an osteotomy has been prepared, a surgeon will use a “guided implant,” which has a unique driver, to ensure proper placement of the implant. However, the Straumann PURE Ceramic Implant currently does not have such a driver; therefore, the surgical guide was used only to prepare the osteotomy while implant placement was performed freehand. Bone quality was determined to be type II. The Straumann PURE Ceramic Implant comes with a separate transfer piece for placement, which snaps into place much like the Tissue Level (Straumann) impression cap. Three dots on the driver line up with a flat surface of the abutment portion of the implant and also indicate distance to the shoulder (1 mm, 2 mm, or 3 mm).
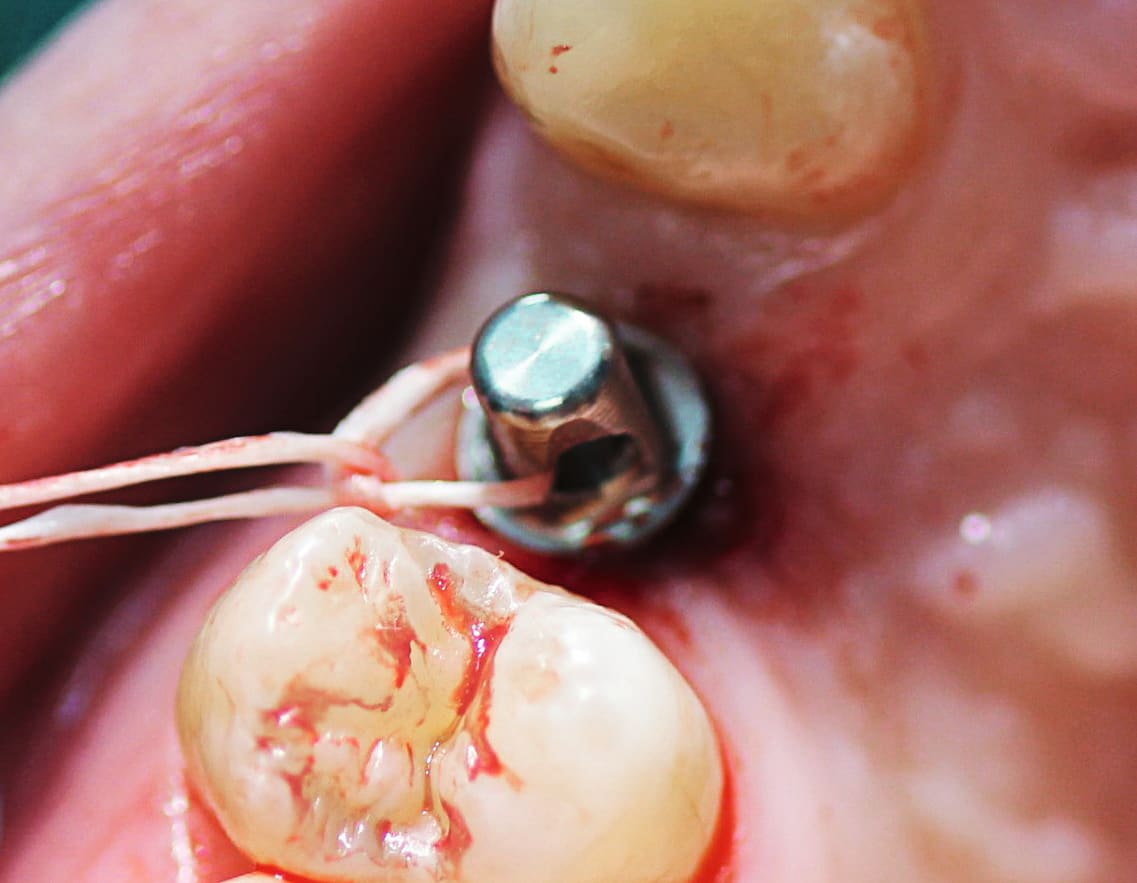
The implant was placed without incidence to the desired depth and position of the dots (Figure 7 through Figure 9). During the healing phase, a protective cap was placed over the abutment to protect it. Because the patient was concerned with esthetics and had a high smile line, it was decided to place a provisional to provide a more esthetic appearance. The recommendation by Straumann not to immediately load a PURE implant3 was taken into account during the DWOS Synergy design session by eliminating occlusal and lateral contacts. Additional modification was then made to the provisional at the time of surgery by further reducing the anatomy and creating more of a custom healing abutment than immediate provisional. The provisional was cemented using temporary cement (Temp-Bond™, Kerr Dental, www.kerrdental.com), and only two interrupted sutures were required to secure the flap.
Final Result
At the 1-week follow-up the tissue was healing beautifully around the implant (Figure 10), and the patient was scheduled for the final impression 7 weeks out. Although the patient said she had been in slight discomfort following the surgery, she stated that this surgery was less painful than the previous extraction. She was pleased to have the modified provisional versus a dark space in her smile.
Conclusion
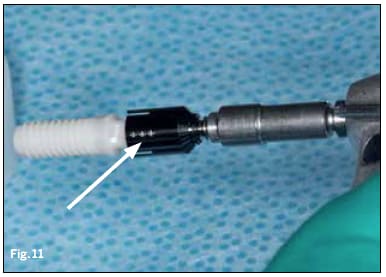
Because the endosteal portion of the Straumann PURE Ceramic Implant is based on the Straumann Bone Level design, the implant does not require additional surgical instruments or drilling protocols for placement, as the specialized transfer piece comes with the implant. When placing the driver onto the Straumann PURE Ceramic Implant abutment care must be taken to align the indicator dots with the facets; otherwise, incomplete seating of the driver may occur (Figure 11).
As implant therapy has evolved, patient expectations have risen. The desire to have a natural-looking, metal-free restoration is growing as evidenced by the decrease of metal substructures for crowns and frameworks and the increase in ceramic restorations.5-7 While titanium can cause a graying of the tissues, the ivory coloring of the Straumann PURE Ceramic Implant can provide a more esthetic outcome. Knowing that her husband had a titanium implant in the anterior region and she could see the gray hues, the patient in this case was gratified to have the option of a Straumann PURE Ceramic Implant.
While all-ceramic implants have the potential to provide improved esthetic outcomes, they do require highly precise planning and placement. Initially, one might consider the ceramic implant used in this case to be limited by design, and to a degree it is; however, using the DWOS Synergy workflow can help reduce the challenge of placing a monotype implant.
About the Authors
Richard Zimmermann, DDS
Associate Professor, Comprehensive Dentistry, School of Dentistry, University of Texas Health Science Center, San Antonio, Texas
Stefanie Seitz, DDS
Associate Professor, Comprehensive Dentistry, School of Dentistry, University of Texas Health Science Center, San Antonio, Texas
Acknowlegment
The authors would like to thank Straumann for their continued support of our programs.
References
1. Kniha K, Gahlert M, Hicklin S, et al. Evaluation of hard and soft tissue dimensions around zirconium oxide implant-supported crowns: a 1-year retrospective study. J Periodontol. 2016;87(5):511-518.
2. Gahlert M, Kniha H, Weingart D, et al. A prospective clinical study to evaluate the performance of zirconium dioxide dental implants in single-tooth gaps. Clin Oral Implants Res. 2016;27(12):e176-e184.
3. Basic information on the surgical and prosthetic procedures – Straumann® PURE Ceramic Implant. Basel, Switzerland: Institut Straumann AG; 2014. https://www.schmidt-dental.pl/wp-content/uploads/2015/11/Straumann_Surgical_Procedures_PURE_Ceramic.pdf. Accessed January 19, 2017.
4. Basic information on Straumann® Guided Surgery. Andover, MA: Straumann USA LLC; 2012. https://www.straumann.us/content/dam/internet/straumann_us/resources/guidemanual/ifu/en/LIT261_Guidedsurgery%20Brochure.pdf. Accessed January 19, 2017.
5. Sorensen JA. The evolution and revolution in prosthodontics continues. Compend Contin Educ Dent. 2016;37(6):402-404.
6. Griffin JD Jr. Conservative zirconia bridge for anterior tooth replacement. Dent Today. 2011;30(11):97-101.
7. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000. 2017;73(1):241-258.