Abstract
As cemented implant restorations have grown in popularity, so has the incidence of peri-implant disease. The association between implant restorations, cement, and this disease, however, remains somewhat unclear. This article examines factors that may contribute to peri-implant disease, including biology, implant depth, restoration depth, and implant material properties, and considers potential causes of the disease involving residual cement. Guidance on how to prevent this problem from occurring is provided.
Dental implants are considered to be highly sophisticated medical devices that provide real value for patients and potential improved quality of life.1 With the introduction of the cemented restoration came the ability to restore implants in a manner similar to the way the natural dentition is treated—namely, crown and bridge prosthetics.2 However, in recent years there appears to have been an increase in the incidence of peri-implant disease, which may be associated in one form or another with this type of restoration.3-6
A recent report by the American Academy of Periodontology includes residual cement as a risk factor for peri-implant disease (perimucositis and peri-implantitis).7 It appears that all implants are susceptible to peri-implant disease.8,9 The purpose of this article is to explore why such a relationship between cements, implants, and disease may exist and to offer guidance related to prevention of the problem.
Contributing Factors
A common misconception is that it is only the type of luting cement used that leads to an implant disease process. However, any cement can lead to destruction around an implant, and even though some cements may present more issues than others, the disease process—as is the case with most diseases—is multifactorial.
In order to prevent peri-implant diseases, it is important to understand factors that contribute to implants being vulnerable to the cement-induced disease process, including biology, depth, environment, implant materials, cement properties, cement application, abutment design, and maintenance. While it is not feasible to discuss all of these issues in one article, several of these topics will be covered.
Clinicians are familiar with dealing with the natural dentition and have, therefore, taken many of the concepts and techniques used when restoring a tooth with a cemented restoration and transferred them to the cemented implant restoration. This must be considered an error, because teeth and implants have very different requirements from each other, as discussed in the following sections.
Biology
Implants have very few similarities to the natural dentition in terms of how they anchor to the alveolar bone and attach to the surrounding soft tissues.8 The periodontium of a healthy tooth has a major advantage over peri-implant tissues in that the existence of supracrestal collagen fibers insert into cementum to hold the soft connective tissues to the tooth. This provides for a robust tooth-to-tissue interface, which limits the ingress of bacteria as well as insult from physical trauma. Also, the arrangement of the periodontal collagen fiber bundles results in compartmentalization that localizes periodontal disease and limits its spread.9 Implants do not have inserting collagen fiber bundles; they do have circumferential fibers that sling around the implant like a halo that in essence provides only one compartment, so any inflammation tends to result in a 360-degree effect. Attachment of the soft tissues to the implant surface is via a hemidesmosomal attachment—considered a weak mechanism that is readily disrupted.
Implant Depth
Implant soft tissues attach at much deeper levels when compared with where the attachment of soft connective tissue exists with the natural dentition. The platform of an implant is frequently related 3 mm below the facial free gingival margin to facilitate an emergence profile of the restoration. Where papillae exist, this relates often to depths closer to 5 mm to 7 mm.10 This is of concern from the standpoint of maintenance because, generally, it is not possible to efficiently clean deeper than 4 mm with scaling instruments11 and because the microbiota at this level tend to favor anaerobic Gram-negative bacteria, which can result in peri-implant disease.
Many of the newly designed, narrower implant systems available today no longer use the free gingival reference marker, and it has been suggested that placement of the head of the implant be 1 mm to 2 mm below the crest of the marginal bone. This further increases implant depth and provides an environment more conducive for these anaerobes.
Restoration Depth
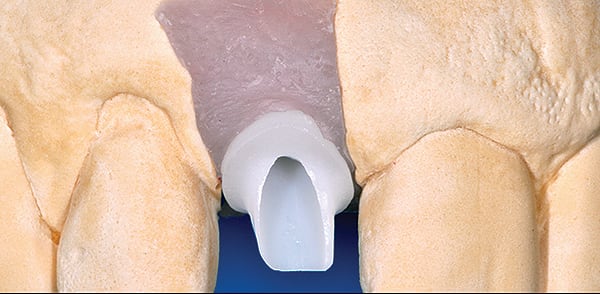
With regards to the natural dentition, cemented restoration margins are recommended for placement not more than 0.5 mm into a healthy gingival sulcus,12 but even that minimal depth is considered a compromise of tissue health. Current clinical recommendations allow clinicians to place cementation margins of implant-supported restorations up to 2 mm subgingivally.13 This is commonly done to hide the abutment–crown interface, to accommodate possible peri-implant tissue recession with time, or to achieve a more natural emergence profile. Linkevicius et al reported on the influence of cementation margin position and the amount of undetected cement;14 they found that all cement remnants could be removed only when supragingival margins existed and concluded that implant abutments should have visible margins for intraoral cementation.
Implant Material Properties
Cements used for the natural tooth must work with enamel and dentin and must be biocompatible with the pulp and surrounding tissues. The main disease associated with cemented natural-tooth restorations is caries resulting from marginal leakage of the luting cement.15 This differs completely from implant cemented restorations, wherein the structural materials of the implant, abutment, or restoration are not biological and so caries does not result.
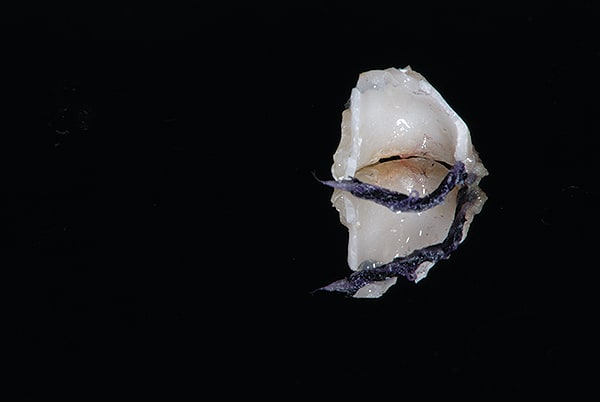
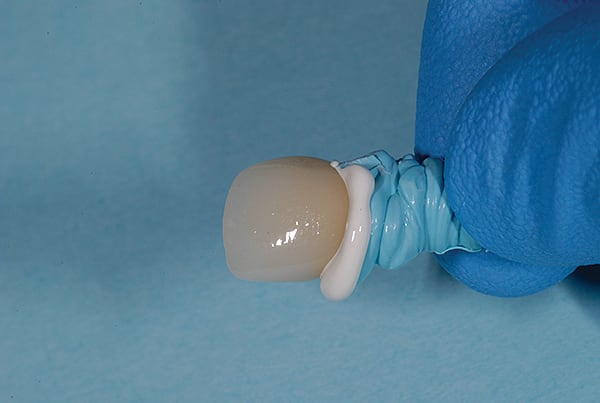
The main biological consideration with cement-retained implant restorations is the vulnerability of peri-implant tissues. Cement contamination readily occurs around implant sites for many reasons. First, as previously mentioned, the soft tissues are much more delicate and vulnerable to tearing when using protection techniques such as retraction cord placement, which is, therefore, contraindicated.16 Cord placement has been shown to strip the weak hemidesmosomal attachment and provide a pathway along the side of the implant for cement extrusion to occur (Figure 1 and Figure 2).17
Second, residual excess cement removal from implants has been shown to be problematic. In 1997 Agar et al showed that even on smooth-surface implant sites, it was not possible to completely remove a resin cement.18 Today’s cements are even more retentive, and the surfaces of implants tend to be purposefully roughened to allow for better healing. Any roughness of an implant or abutment surface must be considered a hindrance to excess cement removal. Retention of any cement remnants may cause an adverse tissue response.
Current Understanding of Peri-implant Disease and Residual Excess Cement
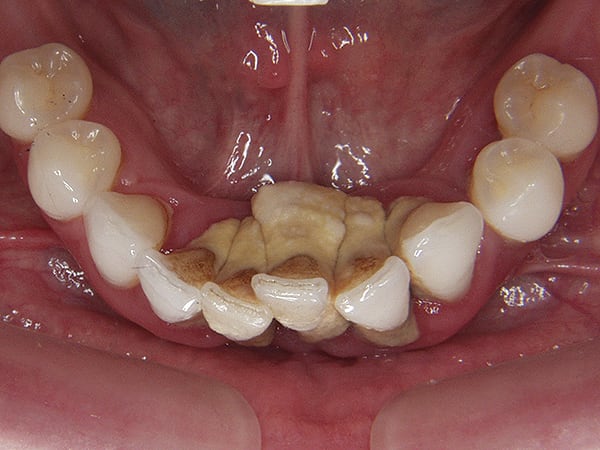
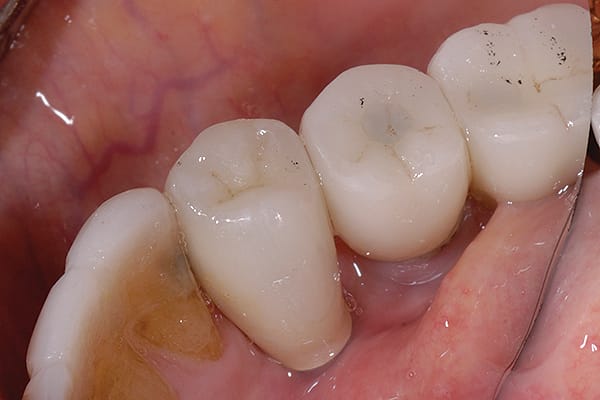
Why implant cement should cause an issue is unclear, as is to what extent the cement plays a part in the process. It is possible that the cement is simply passive and acts as a physical bacterial trap—similar to an overhang on a restoration or calculus effects on the natural dentition (Figure 3). It is also possible that the cement plays a more active role because the destruction of both hard and soft peri-implant tissues is frequently aggressive and extensive (Figure 4 and Figure 5). The disease may be different between patients—and even within the same patient; it may be due to either one major primary factor or a combination of factors.
The author considers there to be at least four potential causes of peri-implant diseases involving residual cement:
1. Microbiological—Wilson5 suggested that the disease process he noted may be microbiological in nature based on the time it took for signs and symptoms to develop, which ranged from 4 months to 9.3 years after the cement-retained implant restoration was placed. Certainly, the environment around implants is conducive to Gram-negative pathogenic bacteria. Depths of 5 mm to 7 mm adjacent to a papilla provide anaerobic sites that allow such potential growth. An ongoing research project that the author is involved in at the University of Washington has recorded variations in the growth patterns of media containing Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, and Porphyromonas gingivalis when exposed to different cements. Initial in-vitro results suggest zinc oxide eugenol cement inhibits both planktonic and biofilm growth to the greatest degree compared with many other cement types.
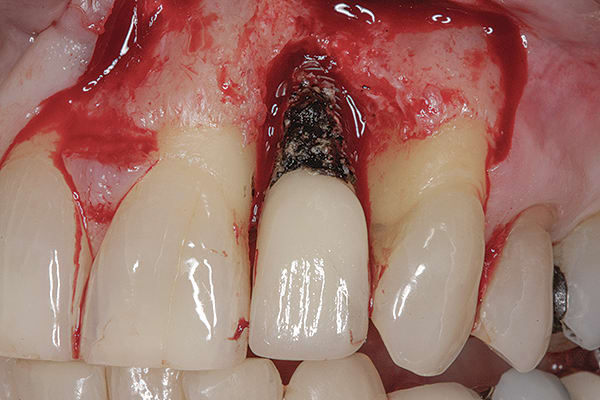
2. Host response: foreign-body reaction—Researchers have evaluated soft tissue removed from inflammatory sites adjacent to dental implants19 and have found foreign-body reactions, some of which included giant cell formation. It is possible that in some cases, the tissue destruction is host-induced as a result of material incorporated within the tissues (Figure 6).
3. Allergic response—It has been reported that some of the newer cements contain allergens such as hydroxyethyl methacrylate (HEMA).20 This material has been identified as causing extreme irritation to tissues—to the extent that material safety data sheets (MSDS) advise that gloves be worn and skin and other mucosal tissue such as eyes be protected when it is used. Because subgingival restorative margins are frequently used with cement-retained implant restorations, complete barrier protection for the soft tissues against chemical insult from cements is rarely, if ever possible. It is quite conceivable that this material is leaching out of the cement prior to setting and producing an immune response.
4. Alterations in implant surfaces—Many cements developed for the natural dentition contain fluoride, which is added to prevent caries when used with a natural-tooth restoration. However, it should be noted that fluoride is a chemical known to etch titanium when used in conjunction with an acid. Some cements clearly state in their instructions that they are not suitable for use with titanium structures, yet it appears many researchers overlook this.21-23 The omission must be considered a critical error. In 2010, Tarica et al24 reported that 17% of US dental schools selected a polycarboxylate as the final cementing media for implant restorations. Durelon™, a popular polycarboxylate, contains fluoride, and a current investigation by the author has shown this material corrodes titanium, resulting in reactive oxidative species that are known to cause inflammation in surrounding tissues.
Implant Luting Cements
There appears to be a lack of consensus in the dental industry regarding implant luting cements. Errors in cement selection have been documented,25 with cements suitable for implant restoration cementation chosen arbitrarily, usually because the clinician is familiar with them and because they are used for natural teeth. Lack of understanding of the requirements may compromise the health of the implant site.
Cement application techniques in the luting of implant-supported crowns have also been scrutinized.26 Techniques appear to be used arbitrarily, with little understanding by clinicians regarding how or where to apply the cement. The practice of cementing restorations on natural teeth has been routinely performed for more than a hundred years with little issue, but very few clinicians have been trained or advised on the most appropriate place to load cement within a restoration. Because few, if any problems have been reported with the natural tooth, most clinicians have little concern for the amount of cement they use. However, implant restoration using cement is very different. A survey of more than 400 dentists reported little, if any, standardization of the amount of cement used.26 Some dentists placed over 30 times more cement than ideally required on the intaglio of the crown before it was seated (Figure 7). Others failed to achieve the minimum amount required (Figure 8).
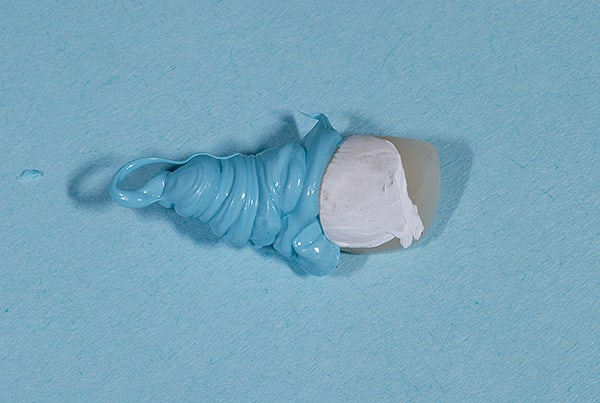
Techniques have been developed to assist clinicians in approximating the right amount of cement.26 For example, pre-extrusion—extraorally with a custom copy abutment—is a quick, easy, and inexpensive method (Figure 9 through Figure 11).
Abutment Design
Margin location is crucial in preventing residual excess cement extrusion into the peri-implant tissues. The work of Linkevicius et al has shown that even if the margin is placed 1 mm subgingivally, cement remnants will be left behind.14
Other solutions to the problem of cement extrusion include providing custom abutments with supragingival margins 360 degrees.27 The use of etchable ceramics that are esthetically compatible with the crown, silanation, and resin bonding reduces the chance of any cement material extrusion and allows for the use of such devices as rubber dam barrier protection,28 which further reduces the possibility of cement extrusion issues (Figure 12 and Figure 13).
The author is currently studying abutment modifications, with a paradigm shift: instead of considering the margin of the abutment–crown as the only site where excess cement can be expressed, the screw-access channel is being contemplated as a repository capable of retaining cement within the system itself.
Simple, inexpensive modification of abutment design that involves changing the way cement flows—by internalizing the flow with internal vents (Figure 14)—also reduces the amount of cement extrusion29 and alters the retentive properties of the cemented restoration.30 With zirconia, whose subtractive material changes may weaken the structure modifications to the screw channel itself, the use of an internal insert device is also being evaluated (Figure 15). The goal, again, is to internalize excess cement and alter flow, which can change the way the system works and which is something that cannot be done with the natural tooth.
Finally, using a screw-retained crown eliminates the residual cement issue completely. Screw-retained restorations have been shown to be highly esthetic and to allow for complete control of the occlusion when fabricated correctly and judiciously (Figure 16 and Figure 17).31
Conclusion
In conclusion, there appears to be an association between implant restorations, cement, and disease. That association, however, is little understood, but many of the inherent problems can be eliminated as long as the implant is not considered equivalent to a tooth. It is paramount that the clinician who is cementing the restoration have a thorough understanding of the processes as they apply to implants. Falling short of such complete understanding could ultimately adversely affect the oral health of patients being treated with this amazing medical device.
About the Authors
Chandur PK Wadhwani, BDS, MSD
Actively involved in implant research at University of Washington, University of California, San Francisco, University of California, Los Angeles, and University of Texas; Private Practice, Bellevue, Washington
References
1. Feine JS, Dufresne E, Boudrias P, Lund JP. Outcome assessment of implant-supported prostheses. J Prosthet Dent. 1998;79(5):575-579.
2. Taylor TD, Agar JR. Twenty years of progress in implant prosthodontics. J Prosthet Dent. 2002;88(1):89-95.
3. Pauletto N, Lahiffe BJ, Walton JN. Complications associated with excess cement around crowns on osseointegrated implants: a clinical report. Int J Oral Maxillofac Implants. 1999;14(6):865-868.
4. Gapski R, Neugeboren N, Pomeranz AZ, Reissner MW. Endosseous implant failure influenced by crown cementation: a clinical patient report. Int J Oral Maxillofac Implants. 2008;23(5):943-946.
5. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80(9):1388-1392.
6. Wadhwani C, Rapoport D, La Rosa S, et al. Radiographic detection and characteristic patterns of residual excess cement associated with cement-retained implant restorations: a clinical report. J Prosthet Dent. 2012;107(3):151-157.
7. Academy report: Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013;84(4):436-443.
8. Albouy P, Abrahamsson I, Persson LG, Berglundh T. Spontaneous progression of peri-implantitis at different types of implants. An experimental study in dogs. I: clinical and radiographic observations. Clin Oral Implants Res. 2008;19(10):997-1002.
9. Albouy JP, Abrahamsson I, Persson LG, Berglundh T. Spontaneous progression of ligatured induced peri-implantitis at implants with different surface characteristics. An experimental study in dogs II: histological observations. Clin Oral Implants Res. 2009;20(4):366-371.
10. Sadan A, Blatz MB, Bellerino M, et al. Prosthetic design considerations for anterior single implant restorations. J Esthet Restor Dent. 2004;16(3):165-175.
11. Stambaugh RV, Dragoo M, Smith DM, Carasali L. The limits of subgingival scaling. Int J Periodontics Restorative Dent. 1981;1(5):30-41.
12. Goldberg PV, Higginbottom FL, Wilson TG. Periodontal considerations in restorative and implant therapy. Periodontol 2000. 2001;25:100-109.
13. Andersson B, Odman P, Lindvall AM, Brånemark PI. Cemented single crowns on osseointegrated implants after 5 years: results from a prospective study on CeraOne. Int J Prosthodont. 1998;11(3):212-218.
14. Linkevicius T, Vindasiute E, Puisys A, et al. The influence of the cementation margin position on the amount of undetected cement. A prospective clinical study. Clin Oral Implants Res. 2013;24(1):71-76.
15. Goodacre CJ, Bernal G, Rungcharassaeng K, Kan JY. Clinical complications in fixed prosthodontics. J Prosthet Dent. 2003;90(1):31-41.
16. Bennani V, Schwass D, Chandler N. Gingival retraction techniques for implants versus teeth: current status. J Am Dent Assoc. 2008;139(10):1354-1363.
17. Wadhwani C, Ansong R. Complications of using retraction cord protection of the peri-implant soft tissues against excess cement extrusion: a clinical report. Implant Realities. 2012;1:20-23.
18. Agar JR, Cameron SM, Hughbanks JC, Parker MH. Cement removal from restorations luted to titanium abutments with simulated subgingival margins. J Prosthet Dent. 1997;78(1):43-47.
19. Ramer N, Wadhwani C, et al. Histologic findings within peri-implant soft tissue in failed implants secondary to excess cement. Report of four cases and review of the literature. New York State Dent J. In press.
20. Nicholson JW, Czarnecka B. The biocompatability of resin-modified glass-ionomer cements for dentistry. Dent Mater. 2008;24(12):1702-1708.
21. Mansour A, Ercoli C, Graser G, et al. Comparative evaluation of casting retention using the ITI solid abutment with six cements. Clin Oral Implants Res. 2002;13(4):343-348.
22. Mehl C, Harder S, Wolfart M, et al. Retrievability of implant-retained crowns following cementation. Clin Oral Implants Res. 2008;19(12):1304-1311.
23. Wolfart M, Wolfart S, Kern M. Retention forces and seating discrepancies of implant-retained castings after cementation. Int J Oral Maxillofac Implants. 2006;21(4):519-525.
24. Tarica DY, Alvarado VM, Truong ST. Survey of United States dental schools on cementation protocols for implant crown restorations. J Prosthet Dent. 2010;103(2):68-79.
25. Wadhwani C, Schwedhelm ER. The role of cements in dental implant success, Part 1. Dent Today. 2013;32(4):74-79.
26. Wadhwani C, Hess, T, Piñeyro A, et al. Cement application techniques in luting implant-supported crowns: a quantitative and qualitative survey. Int J Oral Maxillofac Implants. 2012;27(4):859-864.
27. Wadhwani CP, Piñeyro A, Akimoto K. An introduction to the implant crown with an esthetic adhesive margin (ICEAM). J Esthet Restor Dent. 2012;24(4):246-254.
28. Magne P, Magne M, Jovanovic SA. An esthetic solution for single-implant restorations—type III porcelain veneer bonded to a screw-retained custom abutment: a clinical report. J Prosthet Dent. 2008;99(1):2-7.
29. Wadhwani C. Piñeyro A, Hess, T, et al. Effect of implant abutment modification on the extrusion of excess cement at the crown-abutment margin for cement-retained implant restorations. Int J Oral Maxillofac Implants. 2011;26(6):1241-1246.
30. Wadhwani C, Hess T, Piñeyro A, Chung KH. Effects of abutment and screw access channel modification on dislodgement of cement-retained implant-supported restorations. Int J Prosthodont. 2013;26(1):54-56.
31. Wadhwani C, Piñeyro A, Avots J. An esthetic solution to the screw-retained implant restoration: introduction to the implant crown adhesive plug: clinical report. J Esthet Restor Dent. 2011;23(3):138-143.