Abstract
Achieving accurate impressions of crown and bridge preparations remains a challenge for practicing dentists despite numerous material and technique advances. Precise material placement around the prepared tooth, proper management of soft tissue, and timely dispensing of both wash and tray materials are among the difficulties. As demonstrated in two cases presented, a new pneumatic impression device has been developed as a means of enabling clinicians to create precision impressions more easily, without the need for retraction cord or retraction paste.
Despite advancements in almost every facet of impression-making, from materials to hemostatic agents to cords and retraction paste, achieving accurate impressions of crown and bridge preparations can be frustrating for practicing dentists. A study from Samet and colleagues found that 89% of impressions taken had one or more observable errors; the most common error was voids or tears at the finish line (likely due to difficulty obtaining intimate contact of material around the tooth and gingiva, faulty manipulation of the material while placing it around the tooth, or premature removal from the mouth).1 Creating an exact negative likeness of the patient’s hard and soft tissue, specifically capturing the entirety of the tooth preparation, is essential for positive outcomes.1,2 In three notable editorials, Christensen commented that inadequate impression-making is commonplace and suggested strategies involving careful preparation design and tissue management to improve success.3-5 In each of the editorials, retraction cord is advocated as a means to create a space between the gingiva and tooth in which impression material can be placed to record the margins of the preparation.
Soft-tissue management during crown and bridge procedures is vitally important to making an acceptable impression. Prior to capturing a final impression, practitioners have historically used mechanical retraction through the placement of cord, alone or in combination with a chemical agent (eg, aluminum chloride, ferric sulfate, racemic epinephrine), to promote hemostasis, drying, and retraction of tissue. In one study of North American dentists, it was found that 95% of respondents routinely used gingival retraction cord during crown and bridge impressions.6 To allow enough material to create an undistorted impression, a 0.2-mm space must be created between tooth and gingiva. This minimum space requirement is intended to allow the impression material to flow into the sulcus and not exceed its tear-strength capability, thereby lessening the chance for remnants to be left behind that could cause gingival irritation and inflammation.7-10
It is well known that placing cord creates an acute tissue injury that in some cases can lead to postoperative discomfort, inflammation, and marginal recession.11 Recently, product manufacturers have introduced retraction paste as a less traumatic means of achieving hemostasis and local retraction (eg, Expasyl®, Kerr, www.kerrdental.com; Traxodent®, Premier Dental, www.premusa.com). Even though retraction paste has been shown to create temporary gingival inflammation, it is considered less traumatic compared with placing retraction cord and is as effective in promoting hemostasis.12,13
There are several ways that conventional crown and bridge impressions are taken: the single-step technique using only one material (eg, monophase technique); the single-step technique involving impression materials of two viscosities (eg, light body and heavy body); and the double-step technique, which also includes two materials with different viscosities, however one is allowed to set, followed by placement of the second material as a second step.1,14 Practitioners also have many different tray options for capturing impressions, ranging from closed-bite trays (eg, triple trays), to stock trays, to custom trays (eg, Triad®, DENTSPLY International, www.dentsply.com). Clearly, when combining the choices of impression techniques and trays with materials and cords, the dentist is presented with a potentially confusing array of choices from which to select.
Impression-Making Landscape
Currently in the United States the three main categories of impression materials are polyvinyl siloxane (PVS), polyether (PE), and alginate/alginate alternative. In total, the impression materials market accounts for approximately $275 million in annual sales, the largest percentage of share coming from sales of PVS.15 PVS materials account for approximately half of the total US impression material sales, primarily because of factors such as: fast setting times; several viscosity choices; easy clean-up; pleasant taste; physical properties such as high tear strength, dimensional stability, and excellent elastic recovery; and lower cost than PE.16,17
The procedure of creating a final impression typically involves placing material (usually a low-viscosity material) around the prepared tooth, and filling the impression tray (usually with a higher-viscosity material). To place the material in the sulcus around the prepared tooth, clinicians can use back-filled syringes (eg, Penta™ Elastomer Syringe, 3M ESPE, www.3MESPE.com), 50-mL impression guns, or the digit® Targeted Delivery System (DENTPSLY Caulk, www.caulk.com) consisting of a plastic dispenser and preloaded unit-dose impression cartridges. Filling the impression tray also involves using a 50-mL impression gun or an automatic mixing machine such as a Pentamix™ 3 (3M ESPE) or Duomix™ II (DENTSPLY Caulk).
All of the options for placement of low-viscosity material around the prepared tooth require the dentist to perform two tasks simultaneously: express the material by pushing down on the plunger or squeezing the impression gun, and place the material around the tooth. In addition, if the preparation finish line is modestly subgingival the dentist must create at least 0.2 mm of sulcular width in order to capture an accurate impression. Besides cord, practitioners have also used retraction paste, electrosurgery, lasers, and rotary curettage to create this required space for impression-making.
Three major challenges facing dentists when recording conventional crown and bridge impressions are: placing material precisely around the properly prepared tooth; correctly managing the soft tissue; and dispensing both wash and tray materials into the mouth within allowable working times. The recent introduction of digital impression techniques has changed the dental impression-making landscape; however, according to a recent survey, approximately 75% of dentists are still taking conventional impressions.18-20
A New Delivery Method
A new product, Aquasil Ultra Cordless Tissue Managing Impression Material (TMIM) and digit power™ Dispenser (DENTSPLY Caulk), has been developed as a means to create impressions without the need for retraction cord and to allow easier, precision placement of material. The digit power™ Dispenser is a pneumatic impression device that is compatible with most dental units (Figure 1 and Figure 2). It connects to an air line at the dental chair via commonly available connectors and utilizes digit power™ cartridges. Instead of loading a backfill syringe or squeezing a 50-mL impression gun, with this new system dentists are able to simply step on the rheostat to express material while holding the impression device in a pen grip. The small-diameter intraoral tip on the impression cartridge allows placement directly into the sulcus or around a dental implant. Different sized impression cartridges are available for either single- or multiple-unit cases.
Aquasil Ultra Cordless Tissue Managing Impression Material is designed for use with a single-step, dual-viscosity impression technique. Both the tray material and the wash material for this system have been formulated to provide several advantages compared with Aquasil Ultra (DENTSPLY Caulk). First, because the wash material is intended to be placed around the prepared tooth without the use of cord, it is designed for flow into sulcus widths of less than 0.2 mm without distortion or tearing. Compared with Aquasil Ultra the tear strength of Aquasil Ultra Cordless impression material has been improved to help prevent rips or tears at the impression margin. Additionally, to meet the needs of dentists adopting newer technologies, the materials have been optimized for digital scanners (unpublished data). Figure 3 shows the digit power™ Dispenser being used to capture a final impression (for tooth No. 19).
Case 1
A 25-year-old patient presented complaining that he disliked the appearance of his maxillary central incisors (Figure 4). Intraoral examination revealed multiple resin restorations placed after the area was traumatized during childhood. Once appropriate data were collected, including a complete medical history, radiographs, and periodontal evaluation, the treatment plan was formulated to include lithium-disilicate restorations of teeth Nos. 8 and 9.
Buccal and palatal anesthesia was initiated with 2% Xylocaine® DENTAL with epinephrine 1:100,000 (DENTSPLY Pharmaceutical, www.dentsplypharma.com). The preparations were completed using Two Striper® diamond burs (Premier Dental) to achieve 2 mm of incisal clearance, 1 mm to 1.5 mm of circumferential clearance, and a slightly subgingival butt joint finish line. Care was taken to not traumatize the marginal gingiva; however, to arrest any bleeding, Hemoban (Sultan Healthcare, www.sultanhc.com) was applied on a cotton pledget.
Using a full-arch stock tray, the final impression with Aquasil Ultra Cordless Tissue Managing Impression Material was made without the use of cord (Figure 5). A multi-unit cartridge was used, and the impression was removed from the mouth 4:30 minutes from the start of mixing. After inspection to verify unequivocal capture of the prepared tooth details, provisionals were fabricated and cemented with Integrity® Multi-Cure Temporary Crown and Bridge Material (DENTSPLY Caulk) and Integrity® TempGrip® Cement (DENTSPLY Caulk).
Three weeks later the patient returned for final cementation of crown Nos. 8 and 9. The provisionals were removed and the preparations gently cleaned to remove all of the temporary cement. After fitting and adjusting the crowns, final cementation was achieved with Multilink® Automix (Ivoclar Vivadent, www.ivoclarvivadent.com) translucent shade. All cement was removed with an explorer before final curing, and the margins were polished with a fine composite finishing bur after light-curing of buccal and palatal surfaces for 20 seconds each. Figure 6 shows the completed restorations after cementation.
Case 2
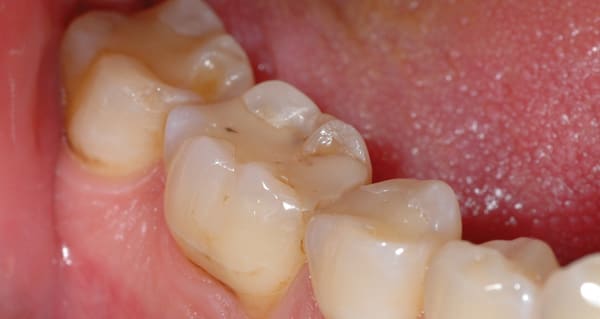
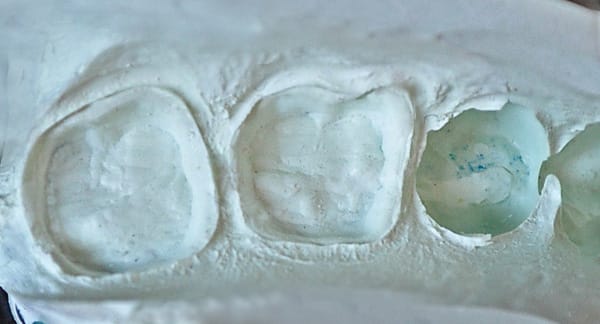
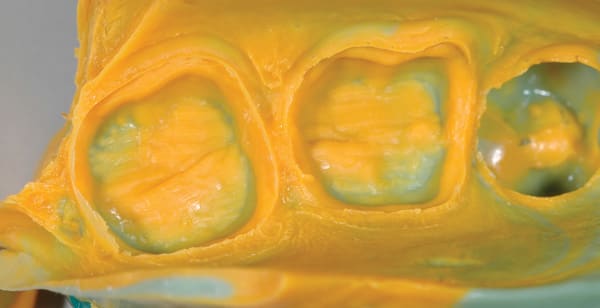
A 40-year-old man presented for crown preparations of teeth Nos. 30 and 31 (Figure 7). The treatment plan involved lithium-disilicate full-coverage restorations. The teeth were prepared as discussed in Case 1 after anesthesia with 4% Articadent™ DENTAL (DENTSPLY Pharmaceutical) containing 1:100,000 concentration of epinephrine. For improved cleansability, the intended location of preparation finish lines was equi- or supragingival; final finish lines extended subgingival on the mesial aspect of both teeth. Two impressions were taken for this case to compare Aquasil Ultra Cordless wash material to Aquasil Ultra XLV wash material dispensed from a 50-mL impression gun. The full-arch stock trays were filled with Aquasil Ultra Cordless tray material and Aquasil Ultra Heavy Body tray material, respectively. Figure 8 and Figure 9 show the two impressions of teeth Nos. 30 and 31; the Aquasil Ultra Cordless impression was taken first, followed by the Heavy Body/XLV impression. While both impressions were clinically acceptable, the first impression made with the Aquasil Ultra Cordless Tissue Managing Impression System was more easily placed and did not require added chair time for tissue retraction.
An important aspect of the Aquasil Ultra Cordless impression material is that errors due to working time/setting time violations are eliminated. Exceeding the working time and/or setting time of an impression material can result in a variety of errors, including incomplete or inaccurate marginal reproduction, tearing, pulls/drags, lack of coadaptation, and others.21 The digit® power™ cartridges are designed so that the entire cartridge can be expressed within the material’s intraoral work time and practitioners will still have enough time to seat the tray properly. The intraoral working time of the single-unit cartridge is 35 seconds while the intraoral working time for the multi-unit cartridge is 1:00 minute; the working time of the tray material is approximately 1:15 seconds.22
Provisionalization of teeth Nos. 30 and 31 was accomplished with Integrity® Multi-Cure and Integrity® TempGrip®. At the final cementation appointment the provisionals were removed and the teeth were cleaned of residual cement with water and pumice. MultiLink® Automix cement translucent shade was used for final cementation. The final case after cementation is shown in Figure 10.
Conclusions
Aquasil Ultra Cordless Tissue Managing Impression System is intended to make conventional crown and bridge impression-making easier and more predictable. Atraumatic impression techniques are not new, nor are powered impression guns.23 The factors that make the Aquasil Ultra Cordless system unique are as follows:
• Precision placement through a fine cannula allows for delivery directly into the sulcus.
• Aquasil Ultra Cordless impression material has been optimized for placement without cord to capture a thin, readable sulcus and prep margins. Increased tear strength reduces the chances that material placed subgingival under pressure will tear or leave remnants behind.
• The working time of the Aquasil Ultra Cordless Tissue Managing Impression Material is designed to coordinate with the amount of material in the unit-dose cartridges for single or multiple preps to reduce impression errors due to violation of working time and setting time. Dentists can now concentrate on one thing—precisely placing the material—rather than squeezing the gun or depressing the impression syringe during the placement step.
• The ergonomically designed digit power™ Dispenser utilizes the pen-grip (the same grip dentists use when using handpieces).
DISCLOSURE
The authors are employees of DENTSPLY Caulk.
ABOUT THE AUTHORS
Jason H. Goodchild, DMD
Clinical Associate Professor, Department of Oral Medicine, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania
Adjunct Assistant Professor, Division of Oral Diagnosis, Department of Diagnostic Sciences, Rutgers School of Dental Medicine, Newark, New Jersey
Research Dentist, Clinical Research and Education, DENTSPLY Caulk, Milford, Delaware
Private Practice, Havertown, Pennsylvania
Nicholas R. Conte, DMD, MBA
Director, Clinical Research and Education, DENTSPLY Caulk, Milford, Delaware
References
1. Samet N, Shohat M, Livny A, Weiss EI. A clinical evaluation of fixed partial denture impressions. J Prosthet Dent. 2005;94:112-117.
2. Paquette JM, Sheets CG. An impression technique for repeated success. Inside Dentistry. 2012;8(2):70-80.
3. Christensen GJ. Have fixed prosthodontic impressions become easier? J Am Dent Assoc. 2003;134(8):1121-1123.
4. Christensen GJ. The state of fixed prosthodontics impressions: room for improvement. J Am Dent Assoc. 2005;136(3):343-346.
5. Christensen GJ. Simplifying and improving soft-tissue management for fixed-prosthodontic impressions. J Am Dent Assoc. 2013;144(2):198-200.
6. Donovan TE, Gandara BK, Nemetz H. Review and survey of medicaments used with gingival retraction cords. J Prosthet Dent. 1985;53(4):525-531.
7. Laufer BZ, Baharav H, Ganor Y, Cardash HS. The effect of marginal thickness on the distortion of different impression materials. J Prosthet Dent. 1996;76(5):466-471.
8. Laufer BZ, Baharav H, Cardash HS. The linear accuracy of impressions and stone dies as affected by the thickness of the impression margin. Int J Prosthodont. 1994;7(3):247-252.
9. Shah MJ, Mathur S, Shah A, et al. Gingival retraction methods in fixed prosthodontics: a systematic review. J Dent Sci. 2008;3(1):4-10.
10. Shiozawa M, Takahashi H, Finger WJ, Iwasaki N. Effects of the space for wash materials on sulcus depth reproduction with addition-curing silicone using two-step putty-wash technique. Dent Mater J. 2013;32(1):150-155.
11. Feng J, Aboyoussef H, Weiner S, et al. The effect of gingival retraction procedures on periodontal indices and crevicular fluid cytokine levels: a pilot study. J Prosthodont. 2006;15(2):108-112.
12. Kazemi M, Memarian MA, Loran V. Comparing the effectiveness of two gingival retraction procedures on gingival recession and tissue displacement: a clinical study. Res J Biol Sci. 2009;4(3):335-339.
13. Smeltzer M. An alternative way to use gingival retraction paste. J Am Dent Assoc. 2003;134(11):1485.
14. Turner JW, Moazzez R, Banerjee A. First impressions count. Dent Update. 2012;39(7):455-458.
15. Strategic Data Marketing website. Impression Materials 2012. www.sdmdata.com.
16. Lawson NC, Burgess JO, Litaker M. Tear strength of five elastomeric impression materials at two setting times and two tearing rates. J Esthet Restor Dent. 2008;20(3):186-193.
17. Kumbuloglu O, User A, Toksavul S, Boyacioglu H. Clinical evaluation of different gingival retraction cords. Quintessence Int. 2007;38(2):e92-e98.
18. Digital Impressions. Dental Lab Products. 2009;34(7):16.
19. Birnbaum N. The revolution in digital impressioning. Inside Dentistry. 2010;6(7):114.
20. Olitsky J. Digital impressioning on its way to becoming mainstream. Compend Contin Educ Dent. 2012;33(9):692-693.
21. Impression Technique Guide. DENTSPLY Caulk. 2005. www.caulk.com/assets/pdfs/Techguide.pdf. Accessed June 17, 2013.
22. Aquasil Cordless Directions for Use. DENTSPLY Caulk.
23. Hoffman JM. Nontraumatic final impressions for fixed partial dentures. J Prosthodont. 1992;1(1):61-64.