Potentially Malignant Lesions of the Oral Cavity
Abstract
Advances in research on potentially malignant lesions of the oral cavity are leading to earlier diagnosis, appropriate treatment, and enhanced follow-up. Knowledge of the terminology associated with these lesions and the clinical warning signs facilitates communication between clinicians, their patients, and other specialists. Upon discovery of a potentially malignant lesion, the clinician should initiate the appropriate investigation, including patient history, biopsy, and histological examination, which will provide a diagnosis associated with the probability of disease progression. Many factors need to be considered when determining the treatment modality, such as the extent of the lesion, location in a high-risk site, the reliability of the patient for follow-up visits, history of previous oral cavity squamous cell carcinoma, habits such as tobacco use, and systemic issues such as immunosuppression.
As advances in research have led to greater understanding of potentially malignant lesions in the oral cavity, clinicians must understand and be able to communicate the relative risks of these lesions, and educate patients on management strategies. This article discusses the terminology, clinical warning signs, and proper steps to take on discovery of an oral potentially malignant lesion.
Terminology
The oral cavity includes the vermilion borders of the lips, labial mucosae, buccal mucosae, buccal vestibulae, alveolar ridges and gingiva, floor of mouth, oral tongue, and hard palate. The most common form of oral cavity cancer is squamous cell carcinoma, which accounts for about 94% of all oral cavity malignancies. Squamous cells are epithelial cells that form the surface covering of the oral mucosa; thus oral cavity squamous cell carcinoma (OCSCC) is a malignancy of surface epithelial cells. A potentially malignant lesion is one that has a greater risk to become an OCSCC than clinically normal oral mucosa.
Precancer
OCSCC occurs as a temporal summation of many molecular and biochemical cellular abnormalities, as well as immune system alterations and changes in the underlying fibrovascular stroma.1 As initial cellular abnormalities accumulate they are reflected in alterations of the clinical appearance of the affected epithelial tissues, known as precancer. Precancer refers to a clinically visible precancerous lesion, which is a benign, morphologically altered tissue that has a greater than normal risk of malignant transformation.2 Precancerous lesions can clinically present as white, red, or mixed white and red changes of the epithelial surface.
Potentially Malignant Lesion
The terminology "precancerous lesion" implies that the lesion will follow an irreversible and inevitable progression to OCSCC. Research shows that, while there is statistical evidence to predict particular clinical and microscopic changes that are associated with development of OCSCC, for any individual lesion clinicians can only estimate the likelihood of progression.3 Thus, rather than precancerous lesion, a term that more accurately reflects current understanding is preferred: potentially malignant lesion.
Precancerous Condition
A precancerous condition is a disease or patient habit that does not necessarily alter the clinical appearance of local tissue, but is associated with a greater-than-normal risk of development of a potentially malignant lesion or cancer in affected tissue.2 A thorough medical, dental, and social patient history is necessary to identify patients raising the index of clinical suspicion for potentially malignant lesions. A history of alcohol or tobacco abuse, multiple oral sex partners, an early age of sexual experience, marijuana use,4 previous OCSCC, and immunosuppression (eg, HIV/AIDS, organ transplantation or bone marrow suppression), are associated with increased risk of OCSCC.
Malignant Transformation Potential
This term is a means of expressing the statistical risk of cancer being present in a potentially malignant lesion or condition, either at initial diagnosis or in the future, usually expressed in percentages.2
Warning Signs
Clinical warning signs of a potentially malignant oral lesion reflect the underlying cellular abnormalities. These clinical changes are generally either white or red in appearance. A white clinical appearance of the epithelium is referred to as leukoplakia, and a red clinical appearance is erythroplakia. Leukoplakia and erythroplakia are strictly clinical terms, meaning “white patch” and “red patch” respectively, and both are diagnoses of exclusion. This means that that the leukoplakia or erythroplakia “cannot be characterized clinically or pathologically as any other disease,” according to the World Health Organization.5
Leukoplakia
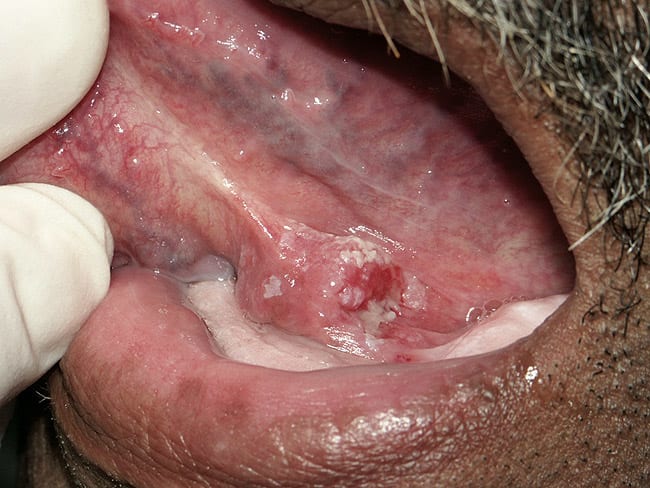
White potentially malignant lesions are far more common than red, however they are less likely to be dysplastic or to transform into OCSCC over time. The challenge is to rule out other potential causes of a clinically apparent white lesion, such as frictional keratosis, hyperplastic candidiasis, morsicatio (chronic nibbling of the tongue, cheek or lip), lichen planus, leukoedema, tobacco pouch keratosis, nicotine stomatitis, and white sponge nevus. A clinical diagnosis of leukoplakia requires that all of these other conditions have been ruled out by clinical history, supplemented by biopsy with microscopic examination as necessary. Microscopically, the diagnosis of a leukoplakia can range from hyperkeratosis without dysplasia, acanthosis (thickening of the surface epithelial layer), epithelial dysplasia (mild, moderate, severe), carcinoma-in-situ, or even squamous cell carcinoma. In one study more than 80% of 3,000 cases of leukoplakia were "without dysplasia."6 This statistic is somewhat site specific, because in certain “high-risk” locations (summarized below) leukoplakias are more often potentially malignant lesions. 6,7
The surface of a leukoplakia may be smooth and homogeneous (Figure 1, Figure 2 and Figure 3), or wrinkled or corrugated with a consistent texture throughout (Figure 4). A non-homogenous leukoplakia may exhibit a papillary surface (verrucous leukoplakia), or areas of erythematous change, which are referred to as erythroleukoplakia (Figure 5). Erythroleukoplakia may present as a broad area of mixed red and white surface change or as uniform small whitish papules on an erythematous base (ie, speckled leukoplakia). Areas of verrucous change, redness or ulceration in a leukoplakia have a greater probability of malignant transformation to OSCC than leukoplakia without these changes. In one study, non-homogenous leukoplakias showed a seven-fold increased risk of malignant transformation when compared to homogenous leukoplakias.8
Erythroplakia
Red potentially malignant lesions are far less common than white lesions, however they are much more likely to be dysplastic or to transform into OCSCC over time.9 Erythroplakia is a flat macule or patch in which the mucosal surface has a red velvety or finely granular surface appearance (Figure 6). The borders of a true erythroplakia are usually well demarcated in contrast to those of an inflammatory process, in which the borders tend to merge with the surrounding normal tissue. Erythroplakic lesions may be symptomatic when patients eat hot or spicy foods and most often occur at high-risk sites for OCSCC (listed below). The major differentials for clinical erythroplakia are inflammatory processes, trauma, or burns, which must be ruled out by clinical history combined with the results of a biopsy that excludes infection, physical or chemical injury, dermatoses or immune reactions.10 The histologic diagnosis of a true erythroplakia may be epithelial atrophy (thinning of the surface epithelial layer), ulceration, epithelial dysplasia, carcinoma-in-situ or squamous cell carcinoma. In one study, 91% of erythroplakic lesions were invasive OCSCC, carcinoma-in-situ, or severe epithelial dysplasia.11
Location, Location, Location
Potentially malignant lesions of the oral cavity are most likely to undergo malignant transformation to OCSCC in the following sites:
- Lower lip vermilion
- Tongue (lateral borders > ventral tongue > base > dorsum)
- Floor of the mouth
- Retromolar trigone / soft palate / tonsillar pillars and palatine tonsils
While the soft palate and palatine tonsils are not technically in the oral cavity proper, they are contiguous with the retromolar trigone and in extensive lesions it may be difficult to determine whether the site of origin was the oral cavity proper or the oropharyngeal tissues. Oropharyngeal tissues that are high-risk sites for oropharyngeal squamous cell carcinoma (OPSCC) are the palatine tonsils, and the lingual tonsils located on the base of tongue. OPSCC is linked to human papilloma virus infection.12 This does not mean that potentially malignant lesions never occur in other oral cavity sites. However, the high-risk sites are those in which these lesions require a more frequent follow-up.
Steps to Take Upon Discovery
Patient History
Some questions that can be useful to investigate an oral abnormality are:
Are you aware of any changes in your mouth?
Symptomatic lesions are often the result of an inflammatory process, while potentially malignant lesions are generally asymptomatic.
If yes, how long have you been aware of these changes?
A long-standing (years to decades) change or one that has not progressed since first discovery is less likely to be a potentially malignant lesion.
Has this ever happened before? Does the lesion come and go or is it always present?
The history of an exacerbating and remitting lesion or one that exhibits a cyclic occurrence is less consistent with a potentially malignant lesion.
Has the lesion become larger since you first noticed it?
Potentially malignant lesions tend to change over time. White lesions may become mixed with a red component or develop a verrucous surface. Lesions may become more extensive or firm or ulcerated, particularly if they undergo malignant transformation.
Is there any discomfort or pain associated with the lesion?
Early potentially malignant lesions tend to be asymptomatic, however development of discomfort or pain may indicate malignant transformation.
Have you started taking any new medications or changed your oral care regimen?
Temporal association of a new environmental factor with the onset of an oral abnormality suggests they might be related. If possible, cessation of the environmental change may be tried to see if the potentially malignant lesion subsides.
Do you use tobacco products or chew betel quid?
Many leukoplakias are associated with tobacco use, and cessation of the habit may result in resolution of the lesion.10
After questioning and clinical examination, a possible cause for a lesion may be determined (eg, contact with a sharp tooth cusp, poorly fitting dental appliances, hypersensitivity to dentifrice or foods, an infectious process, masticatory friction on an edentulous ridge, or trauma). It is important to document and monitor the lesion.13 If it is likely that the lesion is reactive, any appropriate treatment should be instituted and the area should be re-evaluated in 7 to 14 days.14 Fortunately, the vast majority of lesions do resolve after a 2-week period of time. However if the lesion has become larger or changed characteristics, or did not respond to treatment as expected, a biopsy with histologic examination is the appropriate management. It is better to confirm that the lesion is not a potentially premalignant lesion, than to wait until a potentially malignant lesion is malignant.
Biopsy
An incisional biopsy removes a representative portion of the lesion, and is recommended if the lesion is large. When performing a biopsy of erythroleukoplakia, the area of red change should be included in the specimen sent to the pathologist. An excisional biopsy removes the entire clinically visible lesion and is recommended if the lesion is small. Biopsy is avoided only when it could significantly endanger the patient’s health or safety. The result of the biopsy determines what types of follow-up measures are necessary. Only a surgical biopsy of architecturally intact tissue can diagnose potentially malignant lesions and OCSCC.
Hyperkeratosis/Hyperplastic candidiasis
After biopsy, some white lesions may be microscopically determined to be a simple hyperkeratosis (thickening of the superficial keratin layer) with normal epithelial cells (Figure 1). Occasionally the biopsy reveals an infection of the epithelial surface with candidal organisms (Figure 3). Hyperplastic candidiasis often induces an inflammatory response with significant cellular atypia seen microscopically. In these cases, after a course of appropriate anti-fungal therapy, the lesion should be re-evaluated.15 If the lesion has not resolved, a re-biopsy may be necessary to rule out opportunistic candidal colonization of a hyperkeratotic potentially malignant lesion.
Oral epithelial dysplasia and carcinoma-in-situ
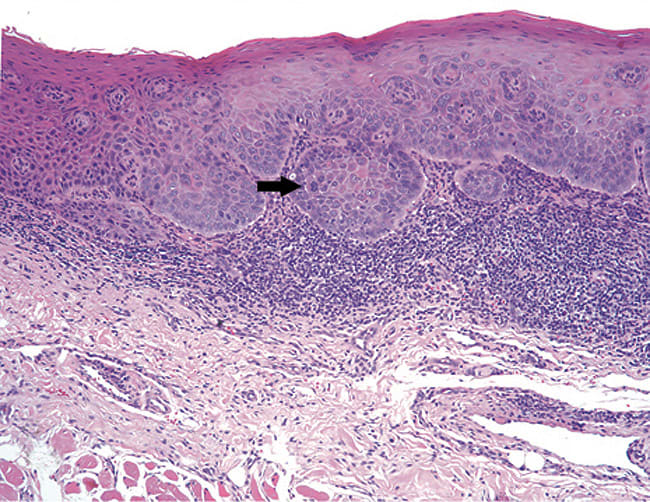
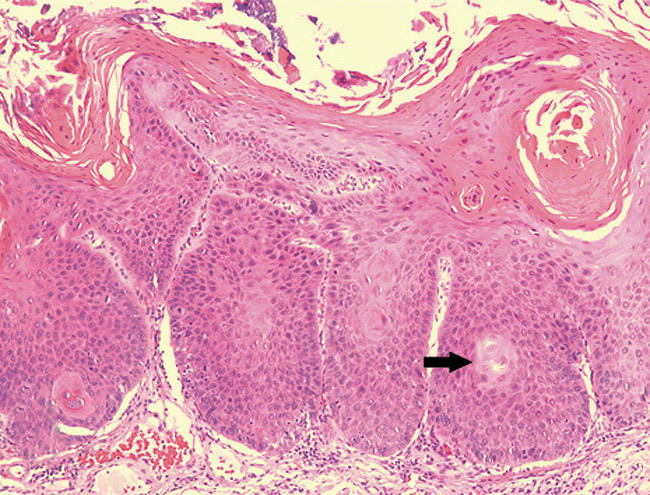
A microscopic diagnosis of epithelial dysplasia means that there are cytologic and/or epithelial architectural abnormalities. A diagnosis of dysplasia will be assigned a category—either mild, moderate or severe.16 Mild epithelial dysplasia is limited to the lower one-third of the epithelial thickness (Figure 7). Moderate epithelial dysplasia is more than one-third but less than two thirds of the epithelial thickness (Figure 8). Severe epithelial dysplasia involves more than two-thirds of the epithelial thickness (Figure 9), while carcinoma-in-situ means that the entire thickness of the epithelium is abnormal (Figure 10), although the malignant cells have not yet become locally invasive. Complicating the prediction of which lesions will undergo malignant transformation, dysplastic lesions may become invasive OCSCC without first exhibiting severe epithelial dysplasia or full-thickness carcinoma-in-situ. In a meta-analysis of pooled data from 992 patients, malignant transformation rate for mild to moderate dysplasia is estimated at 10.3%, while the malignant transformation rate of moderate to severe dysplasia is 24.1%.17
Biopsy Results
A biopsy that is negative for OCSCC should not be viewed as an unnecessary procedure. If the tissue is without dysplasia, that is good news for the patient. In some cases the biopsy reveals a diagnosis such as erythema migrans, hairy leukoplakia, lichen planus or traumatic ulcerative granuloma, which explains the clinical abnormality, and gives a rationale for further treatment. When the histologic diagnosis of a clinical leukoplakia or erythroplakia includes the words “without dysplasia” or “the lesion is benign” this means that periodic clinical observation of any remaining clinical lesion at recall appointments (every 6-12 months) is adequate for management. Of course the patient should be advised to contact the office for an examination if any changes or symptoms occur before the next recall visit. Studies have shown that lesions without dysplasia eventually showed malignant transformation in approximately 2% of cases.8 In the event that the histology is negative for dysplasia, or the severity is less than expected from the extent and characteristics of the clinical lesion, the clinician should not hesitate to contact the pathologist who read the slides and to discuss the need for another biopsy. The possibility of a sampling error is a real concern because oral potentially malignant lesions may be histologically heterogeneous, and a non-representative sample may have been submitted. A pathologist can only report what is seen on the slide, even though the lesion may be highly suspicious clinically. This concept is illustrated by Figure 5.
If dysplasia is noted on the diagnosis line of the biopsy report, that is important information because, while dysplasia is not OCSCC, this tissue has a greater potential for malignant transformation than clinically normal tissue. As noted previously, the malignant transformation rates for dysplasia vary according to severity—an important consideration when planning treatment. Many additional factors need to be considered when determining the treatment modality, such as the extent of the lesion, location in a high-risk site, the reliability of the patient for follow-up visits, history of previous OCSCC, habits such as tobacco, marijuana and/or ethanol abuse, and systemic issues such as immunosuppression. There is currently no evidence of effective nonsurgical treatment in preventing progression of dysplastic lesions to OCSCC.18 In the previously mentioned meta-analysis of data from 992 patients with dysplastic lesions, those who were not treated with surgical excision experienced malignant transformation rates of 14.6%, as compared to patients whose lesions were surgically excised who experienced malignant transformation rates of 5.4%.17 However, it is important to understand that even surgical intervention does not prevent all oral potentially malignant lesions from malignant transformation.8
Conclusion
The terminology associated with potentially malignant lesions of the oral cavity has been summarized to allow better communication between clinicians and their patients, as well as other specialists that may be consulted such as oral pathologists and surgeons. Photographs were presented as examples of the clinical warning signs of oral potentially malignant lesions and the proper steps to take upon discovery of a potentially malignant lesion in the oral cavity have been described. The likelihood of malignant transformation of any individual oral potentially malignant lesion cannot be determined with certainty, although the histologic examination of intact tissue can provide a diagnosis that is associated with a probability of disease progression.
References
1. Lingen MW, Pinto A, Mendes RA, Franchini R, Czerninski R, et al. Genetics/epigenetics of oral premalignancy: current status and future research. Oral Dis. 2011;17 Suppl 1:7-22.
2. Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. St. Louis, MO: Saunders; 2009.
3. Speight PM. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 2007;1(1):61-66.
4. Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type16-negative head and neck cancers. J Ntl Cancer Inst. 2008;100(6):407-420.
5. Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumours: pathology and genetics of head and neck tumors. Lyon, France: International Agency for Research on Cancer Press; 2005.
6. Waldron CA, Shafer WG. Leukoplakia revisited. A clinicopathologic study 3256 oral leukoplakias. Cancer. 1975;36(4):1386-1392.
7. Melrose RJ. Premalignant oral mucosal diseases. J Calif Dent Assoc. 2001;29(8):593-600.
8. Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Long-term treatment outcome of oral premalignant lesions. Oral Oncol. 2006;42(5):461-474.
9. Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52(4):195-215.
10. Wright JM. Oral precancerous lesions and conditions. Semin Dermatol. 1994;13:125-131.
11. Shafer WG, Waldron CA. Erythroplakia of the oral cavity. Cancer. 1975;36(3):1021-1028.
12. Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3(6):78-81.
13. Glick M, Johnson NW. Oral and oropharyngeal cancer: What are the next steps? J Am Dent Assoc. 2011;142(8):892-894.
14. van der Waal I, Axell T. Oral leukoplakia: a proposal for uniform reporting. Oral Oncol. 2002;38(6):521-526.
15. Sitheeque MA, Samaranayake LP. Chronic hyperplastic candidosis/candidiasis (candidal leukoplakia). Crit Rev Oral Biol Med. 2003;14(4):253-267.
16. Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42(10):987-993.
17. Mehanna HM, Rattay T, Smith J, McConkey CC. Treatment and follow-up of oral dysplasia—a systematic review and meta-analysis. Head Neck. 2009;31(12):1600-1609.
18. Brennan M, Migliorati CA, Lockhart PB, Wray D, Al-Hashimi I, et al. Management of oral epithelial dysplasia: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103 Suppl:S19.e1-12.
About the Author
Lynn W. Solomon, DDS, MS
Associate Professor,
Department of Oral & Maxillofacial Pathology
Tufts University School of Dental Medicine
Boston, Massachusetts