Howard E. Strassler, DMD
Most direct restorations placed in restorative dentistry for both posterior and anterior teeth are adhesive composite resins. The chemistry for the majority of composite resins currently used in restorative dentistry is based on Raphael Bowen’s 1962 patent for composite resin, which describes a silane-coated silica glass-reinforced resin using a glass filler particle embedded in a bisphenol A glycidyl methacrylate (Bis-GMA).1 The classification of composite resins in the past decade has included anterior or posterior application specific restorative materials, as well as universal composites for both anterior and posterior clinical applications.2 In deciding which composite resin to use for different clinical applications, the criteria for this universal composite may include:
- multiple shades and translucencies to match the natural dentition
- wear similar to natural tooth structure
- no plastic deformation in function
- a simple placement technique
- minimal shrinkage and minimal contraction stress during polymerization
- high double bond conversion of the monomer chemistry system
- excellent marginal adaptation and sealing
- a radiopacity equal to or greater than enamel and dentin for ease of radiographic evaluation
- a quick, exact, nontooth destructive finishing technique
- chemical compatibility with all adhesive systems2,3
Some deficiencies in clinical performance and postoperative patient complaints with adhesive composite resins relate to problems with resin shrinkage, contraction stresses created by composite polymerization, and gap formation at margins and at the dentin–composite interface during polymerization.4-6 This gap formation caused by resin polymerization shrinkage can contribute to loss of adhesion, bacterial invasion, recurrent caries, postoperative sensitivity, and pain on mastication.7
Polymerization shrinkage and the stresses associated with shrinkage can have a direct impact on the clinical performance of dental composites. During the polymerization kinetics of resin-based composites, the initial double bond concentration of the monomer and degree of conversion achieved during polymerization affect the final shrinkage results—ie, gaps and stresses. These physical properties can directly affect the longevity of composite resin restorations and contribute to marginal sealing and microleakage problems.8-11 Also, the degree of polymerization based on chemical reaction conversion of the double bonds of resin monomers within the composite has been thought to contribute to bioincompatibility.12,13
While filler systems for dental composites have been significantly improved, the principal chemistry still being used with composite resins is based on Bowen’s 1962 patent for Bis-GMA. A review of the literature reveals a number of clinical deficiencies related to Bis-GMA chemistries within composite resins. These deficiencies include inadequate double-bond conversion of the dimethacrylate monomers into polymers, polymerization shrinkage, shrinkage stresses, gap formation between the adhesive and tooth substrate, decreasing physical properties of the composite due to water sorption, water solubility, and solubility to alcohol with chemical softening of the resin and composite wear.2,8,10,11,14-16
In recent years, dental resin polymer chemistry research has focused on substituting the existing composite chemistries with improved resin monomer systems. Researchers at the University of Colorado, led by Jeffrey Stansbury, developed a unique monomer chemistry based on dimer acid monomers that significantly reduced polymerization shrinkage and shrinkage stresses, while increasing the initial double bond concentration of the monomer and degree of double bond conversion achieved during polymerization.11,17 This was accomplished by synthesizing a novel diluent monomer based on a dimer acid chemistry derived from soy, which was radically different from conventional diluents and Bis-GMA resins. Recently, this dimer chemistry conversion technology was incorporated into a new low-shrinkage, high monomer conversion nanohybrid composite resin called N’Durance® (Septodont, www.septodontusa. com).18,19 The volumetric shrinkage of Bis-GMA nanohybrid-based composites ranges from 1.85% to 3%, while the dimer acid chemistry has a reported polymerization shrinkage of 1.27%.18,19 A composite resin requires high radiopacity and wear resistance. With N’Durance, the use of optimized nanofillers of ytterbium fluoride, barium glass, and silica makes this composite easy to distinguish in radiographs and provides for wear resistance similar to existing nanofilled composites.18,19 With this significantly lower volumetric shrinkage and nonstick formulation, well-adapted composite resin restorations are more easily achieved. Side benefits of this new chemistry include extremely low water sorption and solubility, which contributes to color stability (no color shifting), marginal integrity, and stain resistance of the composite. A clinical research study evaluating the N’Durance composite resin demonstrated these results at 1 year: all restorations had excellent anatomic form and marginal adaptation, and showed no marginal discoloration and no surface staining. All restorations were retained, and no evidence of secondary caries was observed.20 No patients exhibited postoperative sensitivity. The researchers concluded the N’Durance composite produced good clinical results for anterior restorations at the 1-year evaluation with no postoperative sensitivity or gingival irritation. This same dimer acid high conversion chemistry is used for the N’Durance Dimer Flow flowable composite resin.
Case Reports
The dimer chemistry nanofilled hybrid composite resin, N’Durance, is classified as a universal composite resin that can be used for both anterior and posterior restorations. Using three different cases, the functional and esthetic applications of N’Durance are illustrated. This dimer acid nanohybrid composite is chemically compatible with both etch-and-rinse and self-etching adhesives. In the cases presented, various etch-and-rinse adhesives were used. The finishing and polishing techniques included finishing burs, finishing discs, silicone polishers, and a polishing brush. In this author’s experience, the final enamel-like luster of the N’Durance composite resin can be achieved using a variety of composite polishing systems. When placing N’Durance restorations, finishing and polishing can be accomplished with the burs, diamonds, and other abrasives already in use.
Case 1
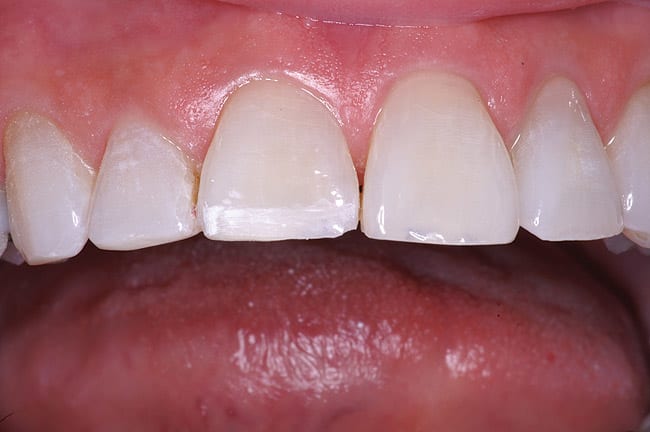
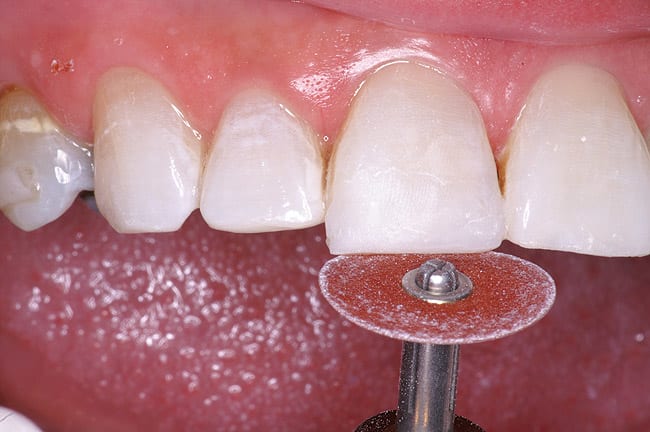
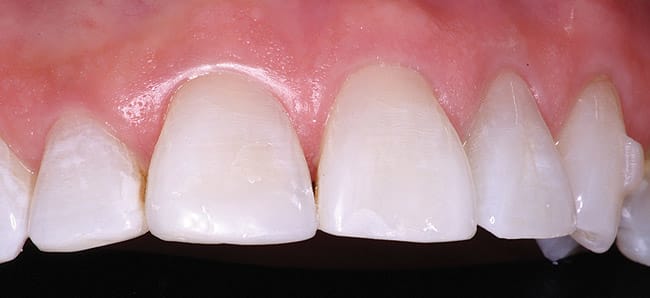
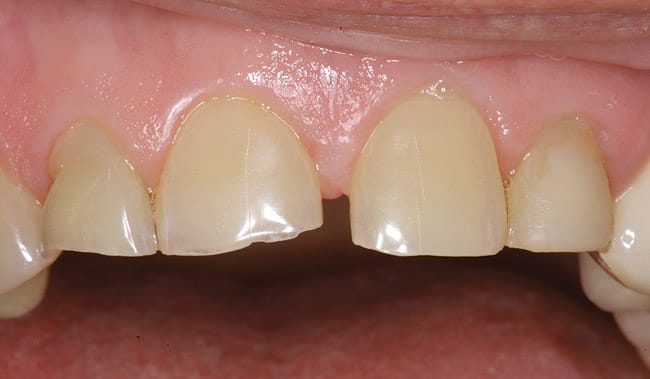
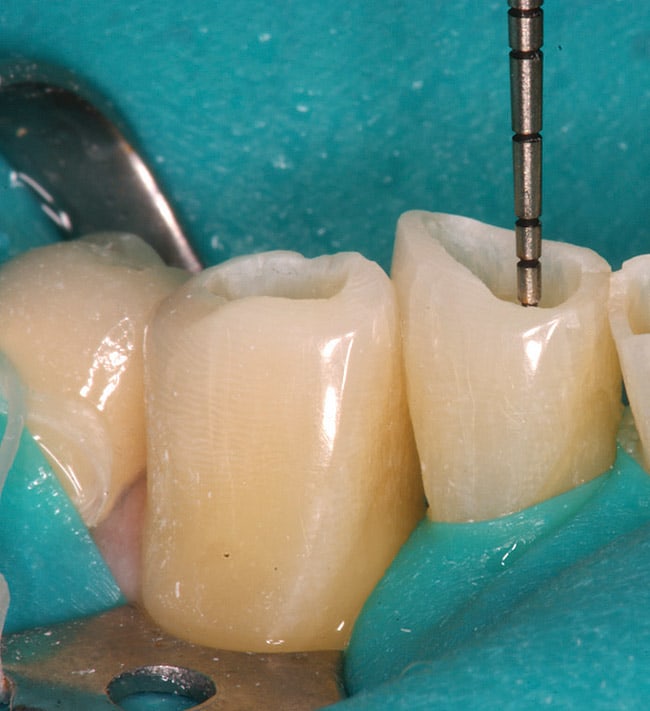
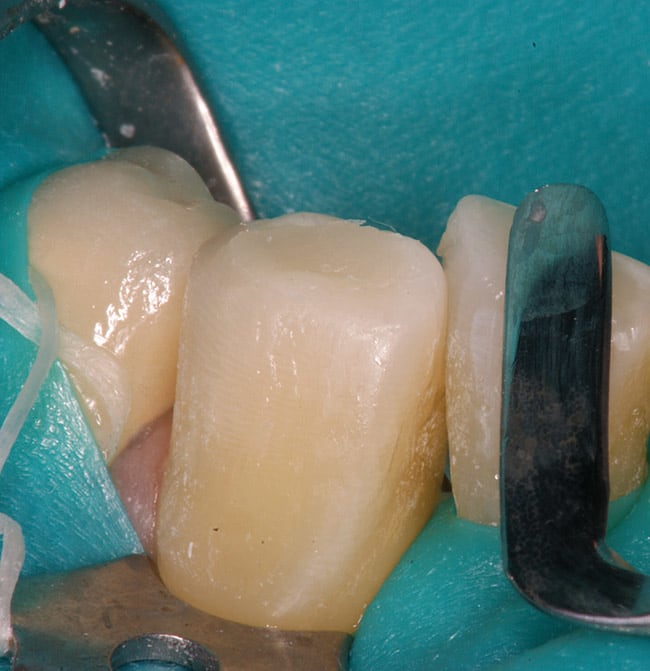
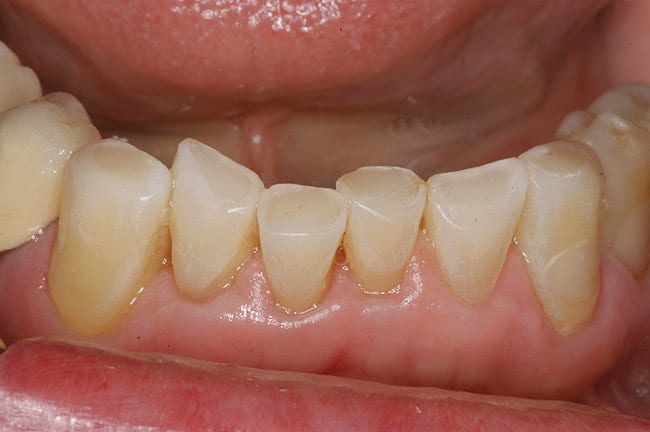
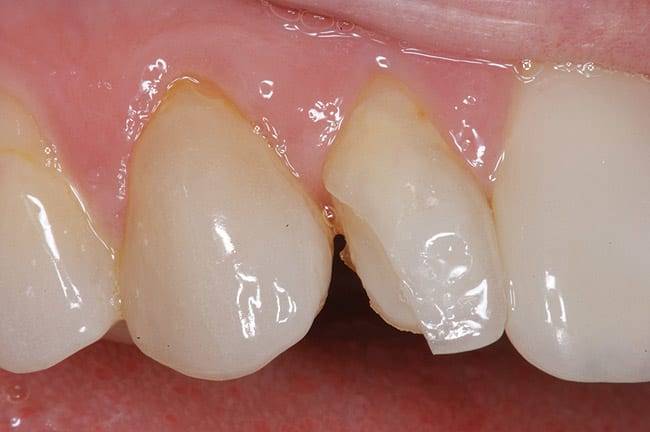
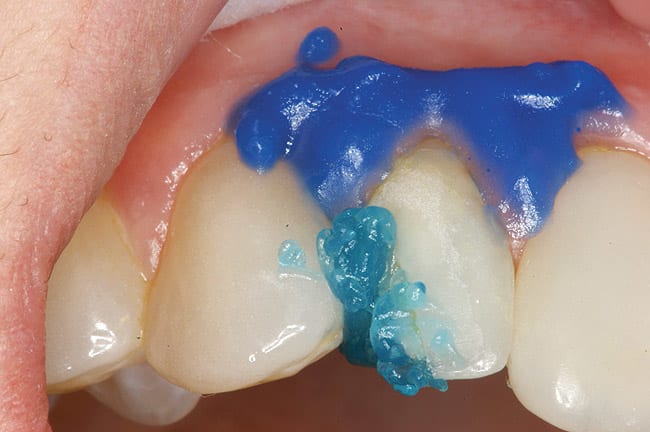
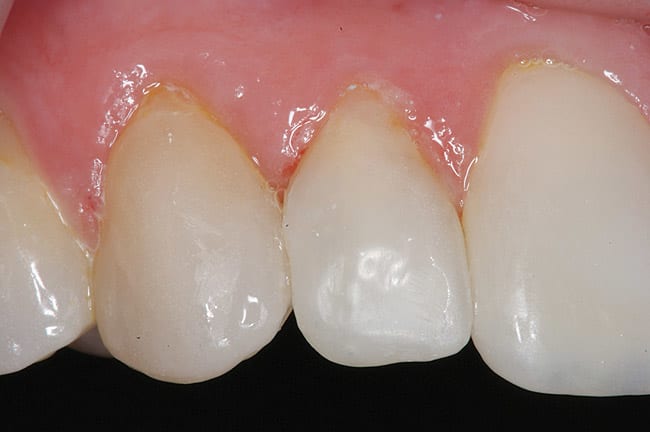
The patient had a chief complaint of a traumatic fracture of the distal incisal edge of the maxillary right central incisor and chipping of the adjacent incisors (Figure 1). The maxillary incisors were reshaped using a coarse zirconia thin plastic-backed abrasive disc (XT-Sof-Lex™ Disc, 3M ESPE, https://www.3mespe.com) (Figure 2). The facial and lingual surfaces of tooth No. 8 were prepared with a 2-mm long intraenamel chamfer with a medium-grit diamond using a high-speed handpiece with air–water spray (Figure 3). The tooth was etched, rinsed, and dried; a fifth-generation adhesive (SeptoBond™, Septodont) was placed. N’Durance composite resin was placed and sculpted to full contour. The full body, moderate-heavy viscosity, and non-sticky formulation of N’Durance facilitated placement. The composite resin was shaped and finished with the coarse disc (Figure 4), followed by a series of finishing burs for the facial and lingual surfaces. One-step polishing of the composite resin and reshaped teeth was accomplished with a rubber-diamond impregnated polisher (Jazz Supreme Polisher, SS White, www.sswhite burs.com). The completed restoration and reshaped teeth fulfilled the patient’s esthetic expectations (Figure 5).
Case 2
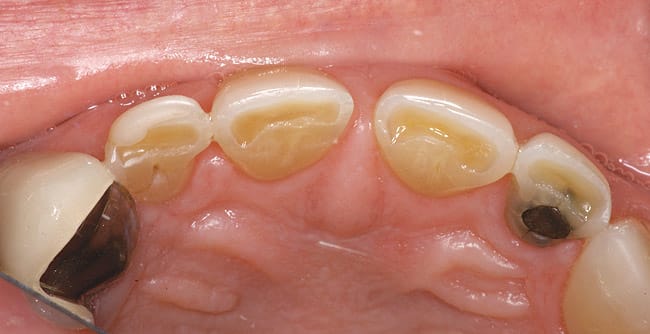
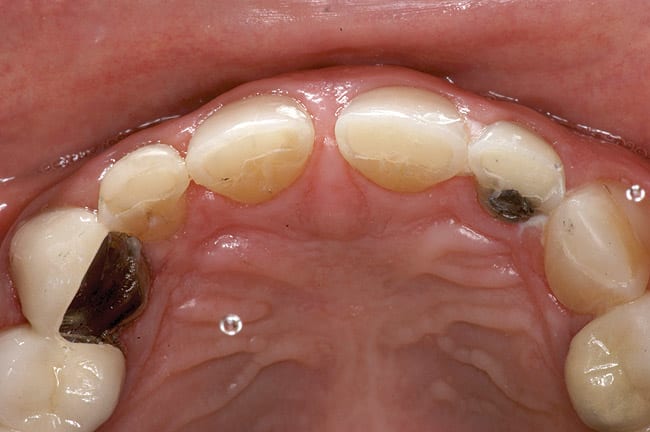
Patients are maintaining their natural dentitions throughout life. With the retention of natural dentition, the clinical condition of attrition of the maxillary and mandibular incisors has become more prevalent. For this patient, the wear of the maxillary and mandibular incisors through the enamel into the dentin was unsightly. The patient also had a chief complaint that his maxillary incisors were becoming shorter ( Figure 6a, Figure 6b, Figure 6c, Figure 6d). For these cases of early attrition into the dentin with minimal tooth length loss, the primary goal of the restoration is to prevent further wear of the incisal edges, using a wear-resistant composite resin without increasing the occlusal vertical dimension. For more severe cases of attrition, a more extensive restoration of the teeth is required. The sequence of treatment in a single visit was the restoration of the mandibular anterior incisal edges for teeth Nos. 22 to 27. Applying a minimally invasive technique with a 12-year success rate for this author, a 329 bur (1 mm in length) was used for an intra dentin preparation of the incisal edges to a depth of 1 mm, leaving an enamel shell intact (Figure 7).21 Usually these preparations can be accomplished without the need for local anesthesia. In the author’s experience, a minimal depth of 1 mm into the dentin, leaving the enamel intact, assures the durability of the restoration. Using an etch-and-rinse adhesive technique, the N’Durance composite resin was placed without changing the height of the mandibular anterior teeth (Figure 8). The composite resin was light-cured, finished, and polished. The maxillary incisors were prepared with the same technique (Figure 9). After etching, rinsing, and drying, then placing a fifth-generation adhesive (Septobond), the composite resin was placed into the intradentin preparations (Figure 10) and adapted to the cavosurface margins. All restorations were finished back to the original incisal heights with a disc and polished with an aluminum oxide impregnated silicone disc (Enhance®, DENTSPLY Caulk, https://www.dentsply.com). Occlusion was verified to ensure that the occlusal vertical dimension was preserved. The restorations provided for function, esthetics, and wear resistance (Figure 11a and Figure 11b ). Impressions were made to fabricate an occlusal appliance to address the patient’s parafunctional occlusal habits.7
Case 3
A patient presented with a traumatic fracture to the distal incisal edge of a porcelain veneer on the maxillary right central incisor (Figure 12). Her father, a dentist, had placed the veneers approximately 2 years earlier and was planning to replace the fractured veneer. To address the patient’s esthetic concerns, a composite resin repair of the distal incisal of the lateral incisor was initiated as an emergency procedure with the knowledge that the veneer would be replaced within several months.
The facial porcelain was prepared with a medium-grit diamond with a high-speed handpiece, air–water spray, and a 2-mm wide margin to provide for removal of the porcelain glaze and adequate retention of the composite resin to the porcelain (Figure 13). The remaining enamel was also lightly prepared with the diamond to remove any residual resin from the enamel surface. The retention of the composite resin restoration included adhesion to the existing enamel from which the porcelain had fractured and adhesion to the facial porcelain. A two-stage etching for restoration was performed: the enamel etched with phosphoric acid and the porcelain with hydrofluoric acid. The gingival tissues were protected with a light-cured resin tissue barrier, and the patient wore protective glasses for the procedure. The porcelain was etched for 2 mins with a buffered viscous 9% hydrofluoric acid designed for intraoral or extraoral etching of porcelain (Porcelain Etch, Ultradent, https://www.ultradent.com). The porcelain etchant was removed from the surface with high-velocity suction before rinsing the surface to avoid accidentally rinsing the porcelain etchant into the oral cavity. The minimal amount of porcelain etchant remaining was rinsed from the surface, and the surface dried. The enamel was etched, rinsed, and dried (Figure 14). The etched porcelain surface was treated with a silane ceramic primer for 30 secs and then dried. A fifth-generation resin adhesive was painted on the enamel and porcelain surfaces, air-thinned, and light-cured. N’Durance dimer nanohybrid composite resin was placed (Figure 15) and shaped, then light-cured. The restoration was finished and polished as previously described. The porcelain repair of the fractured veneer with composite resin provided the patient with an esthetic emergency repair (Figure 16).
Conclusion
In another 2 years, it will be the 50th anniversary of Bowen’s patent on composite resin using Bis-GMA resin and other monomer diluents with a silane-treated glass filler particle. In the past four decades, composite resins have advanced with improvements in size and type of glass fillers. Most composites today are using the original chemistry, with problems of poor conversion of the double bond dimethacrylate monomers into polymers, polymerization shrinkage, shrinkage stresses, water solubility, and solubility to alcohol. Recently, a unique monomer chemistry that significantly reduces polymerization shrinkage and increases the initial double bond concentration of the monomer and the degree of conversion achieved during polymerization was commercialized as a new low-shrinkage high monomer conversion universal anterior– posterior composite resin, N’Durance. Unlike other new composite chemistries that limit their use to specific bonding systems, N’Durance can be used successfully with all existing etch-and-rinse and self-etch adhesives. These changes from the current chemistries appear to be promising.
Acknowledgments
Thank you to Erin Ladwig and Dima Ghunaim who provided assistance in the treatment of cases.
About the Author
Howard E. Strassler, DMD
Professor and Director of Operative Dentistry
Department of Endodontics, Prosthodontics and Operative Dentistry
University of Maryland Dental School
Baltimore, Maryland
References
1. Bowen RL. Silica-resin direct filling material and method of preparation. US Patent 3066112. November 27, 1962.
2. Sensi LG, Strassler HE, Webley W. Direct composite resins. Inside Dentistry. 2007;3(7):76-79.
3. Strassler HE, Goodman HS. Restoring posterior teeth using an innovative self-priming etchant/adhesive system with a low-shrinkage hybrid composite resin. Restorative Quarterly. 2002; 5(2):3-8.
4. Uno S, Shimokobe H. Contraction stress and marginal adaptation of composite restorations in dentinal cavity. Dent Mater J. 1994;13(1):19-24.
5. Van Meerbeek B. Mechanism of resin adhesion: dentin and enamel bonding. Functional Esthetics & Restorative Dentistry. 2008; 2(1):18-25.
6. Strassler HE, Sensi LG. Applications of etch-and-rinse adhesive bonding for esthetic restorative dentistry. Functional Esthetics & Restorative Dentistry. 2008;2(1):26-32.
7. Terry DA. Mastering the technique of direct posterior composite resins. Cont Esthet Rest Pract. 2001;5(6):14-26.
8. Ferracane JL. Using posterior composites appropriately. J Am Dent Assoc. 1992;123(7):53-58.
9. Peutzfeldt A. Resin composites in dentistry: the monomer systems. European J Oral Sci. 1997;105(2):97-116.
10. Gerdolle DA, Mortier E, Droz D. Microleakage and polymerization shrinkage of various polymer materials. J Dent Child. 2008;75(2):125-133.
11. Ge J, Lemon MT, Lu H, et al. Dimer acid-derived dimethacrylates as diluents monomers in restorative resins [abstract 1470]. J Dent Res. 2005(special issue A);84.
12. Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig. 2008;12(1):1-8.
13. Polydorou O, König A, Hellwig E, et al. Long-term release of monomers from modern dental-composite materials. Eur J Oral Sci. 2009;117(1):68-75.
14. McKinney JE, Wu W. Chemical softening and wear of dental composites. J Dent Res. 1985;64(11):1326-1331.
15. Yap AU, Chew CL, Ong LF, et al. Environmental damage and occlusal contact area wear of composite restoratives. J Oral Rehabil. 2002;29(1):87-97.
16. Powers JM, Sakaguchi RL. Craig’s Restorative Dental Materials. 12th ed. St. Louis, MO: Mosby; 2006:189-212.
17. Lu H, Newman SM, Bowman CN, et al. Dimer acid derived dimethacrylate for ternary dental restorative resins [abstract 32]. J Dent Res. 2006(special issue A);85.
18. Bracho-Troconis C, Rudolph S, Boulden J, et al. characterization of a new dimer acid based resin nano-hybrid composite [abstract 81]. J Dent Res. 2008(special issue A);87.
19. Bracho-Troconis C, Rudolph S, Garnhart A, et al. New low-shrinkage dimer acid based microhybrid composite physical properties [abstract 1290]. J Dent Res. 2007(special issueA);86.
20. Ritter H, Lee SS. Clinical evaluation of N’Durance nano-dimer conversion technology dental composite [abstract 1006]. J Dent Res. 2009(special issue A);88.
21. Strassler HE, Serio CL. Conservative treatment of the worn dentition with adhesive composite resin. Dent Today. 2004; 23(8):79-83.