Hui Lu, PhD; Marianela Trujillo-Lemon, PhD; Junhao Ge, PhD; and Jeffrey W. Stansbury, PhD
Abstract: Incomplete polymerization, volumetric shrink age, and shrinkage stress are among the primary disadvantages of current resin-based dental composites. Generally, any attempt to increase final double bond conversion only exacerbates polymerization shrinkage and stress. The use of dimer acid-derived dimethacrylate (DADMA) monomers in novel dental resin formulations is examined in this article as a potential means to address these disparate goals. A series of high molecular weight DADMA monomers with different functional groups used to connect the C36 dimer acid core and the methacrylates were formulated with urethane dimethacrylate (UDMA) and/or ethoxylated bisphenol A dimethacrylate (Bis-EMA) at various compositions to manipulate comonomer compatibility and polymeric mechanical properties. Along with reaction kinetics, dynamic polymerization shrinkage and shrinkage stress were assessed. Specific DADMA monomers demonstrated limited miscibility with either Bis-EMA or UDMA. Appropriate ternary resin formulations produced homogeneous monomeric mixtures capable of controlled polymerization-induced phase separation (PIPS) to yield heterogeneous final polymers. Reduced polymerization shrinkage and stress along with higher conversion was observed for DADMA ternary systems compared with a bisphenol A glycidyl methacrylate (Bis-GMA)/triethylene glycol dimethacrylate (TEGDMA) resin control. The PIPS process resulted in a modest volume recovery and stress relaxation in the later stages of polymerization. These results indicate that certain dimer acid-derived dimethacrylates possess the potential to replace TEGDMA as a reactive diluent in dental resins that display a favorable and unique combination of properties.
While many monomers and oligomers havebeen investigated for use in dental restorative composites, bisphenol A glycidyl methacrylate (Bis-GMA) and urethane dimethacrylate (UDMA) remain the most common monomers employed in resin-based dental restoratives. Because of the relatively high viscosities of these monomers, significant amounts of a reactive diluent comonomer, such as triethylene glycol dimethacrylate (TEGDMA), are included in most resin formulations to adjust handling properties and improve conversion. While a great deal of attention has been directed toward resin-based dental restorative materials that exhibit reduced shrinkage and stress levels during polymerization with practical progress evident, little work has been focused on the issue of limited conversion values, which range from less than 50% to approximately 65% for commercial methacrylate-based composites. For a typical Bis-GMA/TEGDMA resin cured to 60% conversion, more than 15% by mass of the resin remains as free monomer in the polymer,1 which raises concerns about biocompatibility as well as stability of the physical and mechanical polymer properties. With respect to polymerization shrinkage reduction, several basic approaches can be considered. Shrinkage associated with polymer formation directly depends on: 1) degree of conversion; 2) molar shrinkage coefficient; and 3) the initial reactive group concentration. Because of the many clinically noncompromisable properties (including modulus, strength, toughness, and hardness) that are directly determined by the degree of conversion of the resin phase during composite curing, reductions in conversion as a means to limit shrinkage should not be considered a practical option. The shrinkage coefficient is specific to the type of monomer chemistry, and for most methacrylate-based monomers, it is fixed.2,3 The development of the silorane resin chemistry by 3M™ ESPE™,4 as well as the use of thiol-ene step-growth polymerization in dental resins,5,6 are examples of materials that offer lower molar shrinkage coefficients as compared to methacrylate monomers. However, reliance on alternative reaction mechanisms to achieve a reduced shrinkage coefficient may also necessitate revised bonding and filler surface treatment procedures, as well as introduce unconventional polymerization behavior. This suggests opportunities for the development of new methacrylate-based monomers with low initial reactive group concentrations as a means to low-shrinkage dental resins.
Objective
The aim of this study was to formulate and evaluate novel dental resins based on C36 dimer acid core-structured dimethacrylate monomers, which in turn are derived from a natural source of linoleic acid. A straightforward characterization of conversion, polymerization shrinkage, and polymerization stress was the initial basis of this work; however, this study was expanded to include an examination of the effect of heterogeneous polymer structure on the evolution of polymerization shrinkage and stress.
Materials and Methods
The dimer acid-derived monomers—DADMA I, II, and III (Figure 1)—were prepared as previously described.7 The DADMA monomers were formulated in various compositions with UDMA and/or ethoxylated bisphenol A dimethacrylate (Bis-EMA). A control resin composed of 2,2-bis[4-(2-hydroxy-3-methacryloxyprop-1-oxy)phenyl]propane (Bis-GMA) and TEGDMA at a 7:3 mass ratio was used. All the commercial monomers were donated by Esstech Inc (https://www.esstechinc.com). Camphorquinone (0.3 wt%) (Sigma-Aldrich®, https://www.sigmaaldrich.com) and ethyl 4-dimethylaminobenzoate (0.8 wt%) (Sigma-Aldrich) were used as the photoinitiator system, which was activated by 60 secs of visible light exposure at 500 mW/cm2 from a dental curing unit (VIP™, Bisco, https://www.bisco.com).
Real-time photopolymerization kinetics was monitored by near-infrared spectroscopy (Nexus 670, Thermo Fisher, https://www.thermofisher.com). A linometer (Academisch Centrum Tandheelkunde Amsterdam, https://www.acta.nl) was employed to measure the polymerization volumetric shrinkage. Dynamic polymerization stress was measured with a cantilever beam tensometer (American Dental Association Health Foundation, https://www.ada.org), and flexural strength and modulus were obtained in three-point bending with 2 mm x 2 mm x 25 mm specimens on a 20-mm span at a crosshead speed of 1 mm/min (Mini Bionix®, MTS Systems, https://www.mts.com).
Results and Discussion
The DADMA monomers have molecular weights of 673 g/mol to 849 g/mol with initial methacrylate group concentrations of 2.4 mol/L to 2.7 mol/L as compared with the values for TEGDMA of 286 g/mol and 7.5 mol/L, respectively. The DADMA homopolymers have been shown to undergo rapid, high conversion photopolymerization to produce extremely hydrophobic rubbery polymers.7 Monomer I has no hydrogen bond donor functionality and presents the lowest viscosity in the DADMA series. It has limited miscibility with Bis-GMA or UDMA but is compatible with Bis-EMA. Conversely, DADMA monomers II and III, which do form hydrogen bonds through the hydroxyl or urethane functionality, respectively, are compatible with Bis-GMA or UDMA but only partially miscible with Bis-EMA. Therefore, while selected binary comonomer mixtures involving the DADMA monomers and conventional dimethacrylates could be prepared as homogeneous mixtures, ternary compositions that combined one DADMA monomer with both Bis-EMA and UDMA allowed formulations to be prepared in which the degree of thermodynamic compatibility could be precisely tuned. Formulations near the thermodynamic stability boundary as monomers were found to undergo a polymerization-induced phase separation (PIPS) to generate heterogeneous polymer networks. The extent of phase separation depends on the resin composition and reaction conditions with greater sensitivity apparently related to reaction temperature as opposed to reaction kinetics. The final heterogeneous polymers obtained through the PIPS process were slightly to moderately hazy in appearance. The optical transmission efficiency passes through a minimum during polymerization (data not shown here) primarily because of refractive index changes associated with different reaction rates in the different phases.
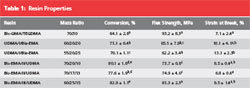
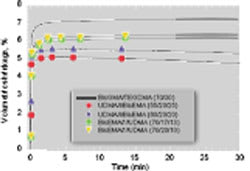
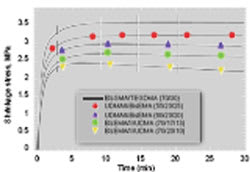
Conversion values of all the experimental resins were significantly greater than the control (Table 1). The flexural strength of the experimental resins was less than that of Bis-GMA/TEGDMA with the exception of the resin based on the urethane-containing monomer III, which notably contained the highest concentration of DADMA monomer used in this study. The experimental resins were generally less brittle than the control. The polymerization shrinkage results for various resin formulations are shown in Figure 2. It is evident that despite the greater limiting conversion values associated with the DADMA-based resins, polymerization shrinkage is significantly reduced. In addition, the shrinkage profiles for some of the heterogeneous experimental materials demonstrate a modest volume recovery during the postcure observation interval. This type of novel behavior has been reported for other systems involving PIPS.8-10 In a similar manner, the experimental resins display lower polymerization shrinkage stress compared to the control (Figure 3), and a modest stress relaxation contribution during the postcure is evident in all the DADMA resins.
Conclusions and Clinical Implications
The introduction of dimer acid dimethacrylate monomers as alternative diluent comonomers is a viable method to reduce the initial reactive group concentration while simultaneously raising final conversion and reducing polymerization shrinkage and shrinkage stress. Based on limited compatibility with conventional dental comonomers, binary and ternary compositions containing dimer acid monomers can undergo controlled phase separation with an additional reduction in polymerization shrinkage and stress—a result of the heterogeneous polymer morphology. Practical methacrylate-based restorative materials that exhibit reduced polymerization shrinkage stress may provide more reliable bonding to tooth structure, while the high degrees of polymeric conversion may enhance long-term material stability while minimizing leachable monomer.
Disclosure
This research was supported by NIH/NIDCR R01DE 014227 and Septodont.
References
1. Stansbury JW, Dickens SH. Network formation and compositional drift during photo-initiated copolymerization of dimethacrylate monomers. Polymer. 2001;42(15):6363-6369.
2. Dewaele M, Truffier-Boutry D, Devaux J, et al. Volume contraction in photocured dental resins: The shrinkage-conversion relationship revisited. Dent Mater. 2006;22(4):359-365.
3. Patel MP, Braden M, Davy KW. Polymerization shrinkage of methacrylate esters. Biomaterials. 1987;8(1):53-56.
4. Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005;21(1):68-74.
5. Cramer NB, Couch CL, Schreck KM, et al. Investigation of thiol-ene and thiol-ene-methacrylate based resins as dental restorative materials. Dent Mater. 2010;26(1):21-28.
6. Lu H, Cariosia JA, Stansbury JW, Bowman CN. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent Mater. 2005;21(12):1129-1136.
7. Trujillo-Lemon M, Ge J, Lu H, et al. Dimethacrylate derivatives of dimer acid. J Polym Sci A Polym Chem. 2006;44(12):3921-3929.
8. Velázquez R, Ceja I, Guzmán J, Castano VM. Morphology-composition-processing relationships in poly (methyl methacrylate)-polytriethylene glycol dimethacrylate shrinkagecontrolled blends. J Appl Polym Sci. 2004;91(2):1254-1260.
9. Schroeder WF, Borrajo J, Aranguren MI. Poly (methyl methacrylate)-modified vinyl ester thermosets: Morphology, volume shrinkage, and mechanical properties. J Appl Polym Sci. 2007;106(6):4007-4017.
10. Cao X, Lee LJ. Control of shrinkage and residual styrene of unsaturated polyester resins cured at low temperatures: I. Effect of curing agents. Polymer. 2003;44(6):1893-1902.
|
Figure 1 Structures of the dimer acid dimethacrylate monomers. |
Table 1 Within each column, the letters indicate statistically significant differences (P < .05) as determined by a one-way ANOVA and Tukey post-hoc pairwise comparison test. |
||||||
|
Figure 2 Dynamic polymerization shrinkage of control and experimental resins. Compositions are based on mass ratios, and all samples (n = 3) were photocured at 500 mW/cm2 for 60 secs at 23° C. |
Figure 3 Dynamic polymerization shrinkage stress of control and experimental resins. Compositions are based on mass ratios, and all samples (n = 3) were photocured at 500 mW/cm2 for 60 secs at 23° C (irradiation started at 30 secs). |
||||||
|
|||||||