Cora Bracho-Troconis, PhD; Marianela Trujillo-Lemon, PhD; Jordan Boulden, BSc; Nicolas Wong, BSc; Kristin Wall, BSc; and Kristina Esquibel, BSc
Abstract: High molecular weight dimethacrylate systems within composite resins present a number of clinical deficiencies, including insufficient monomer conversion, polymerization shrinkage, and polymerization stresses. This study aimed to determine physical and chemical properties of a new high monomer conversion nanohybrid composite resin based on nano-dimer technology (N’Durance® ), compared to other principal products on the market. Specimens were polymerized using a visible light lamp following the manufacturers’ instructions. Compressive and flexural strengths, flexural modulus, water sorption, and tensile strength were determined. Water absorption and solubility were measured. Monomer conversion, polymerization shrinkage, and polymerization stress were calculated. It was shown that products using conventional resin (Bis-GMA/TEGDMA) have an average conversion of 60% for the microhybrids and 50% for the nanofilled composites. The shrinkage stress and contraction with N’Durance are lower than most commercial products: shrinkage contraction was reduced from 2.3% (average of the regular composite) to 1.5%, and the shrinkage stress from 2.5% to 1.1%, with an increase of approximately 27% in the monomer conversion. For products that use conventional resin, volumetric shrinkage increases when the final double bond conversion is increased to reduce the unreacted monomers; however, N’Durance shows high conversion and low polymerization shrinkage.
Abstract: High molecular weight dimethacrylate systems within composite resins present a number of clinical deficiencies, including insufficient monomer conversion, polymerization shrinkage, and polymerization stresses. This study aimed to determine physical and chemical properties of a new high monomer conversion nanohybrid composite resin based on nano-dimer technology (N’Durance® ), compared to other principal products on the market. Specimens were polymerized using a visible light lamp following the manufacturers’ instructions. Compressive and flexural strengths, flexural modulus, water sorption, and tensile strength were determined. Water absorption and solubility were measured. Monomer conversion, polymerization shrinkage, and polymerization stress were calculated. It was shown that products using conventional resin (Bis-GMA/TEGDMA) have an average conversion of 60% for the microhybrids and 50% for the nanofilled composites. The shrinkage stress and contraction with N’Durance are lower than most commercial products: shrinkage contraction was reduced from 2.3% (average of the regular composite) to 1.5%, and the shrinkage stress from 2.5% to 1.1%, with an increase of approximately 27% in the monomer conversion. For products that use conventional resin, volumetric shrinkage increases when the final double bond conversion is increased to reduce the unreacted monomers; however, N’Durance shows high conversion and low polymerization shrinkage.
Today, more than 40 years after composite materials were invented, manufacturers employ the same chemistry for the resin matrix: high molecular weight dimethacrylate systems. Although easy to use, these materials present several problems: first, volume shrinkage and shrinkage stress occurring during polymerization, which results in tooth–composite adhesive failure reducing the longevity and utility of current dental composites. In addition, poor conversion of the dimethacrylate monomers into polymer not only contributes to insufficient wear resistance and mechanical properties, but also jeopardizes the biocompatibility of the composites due to the leachable unreacted monomers. To achieve improved polymeric dental restorative materials, research has been focused mainly on the development of new monomers by increasing monomer conversion without losing the other properties.1-3
A new light-activated low-shrinkage composite (N’Durance®, Septodont, https://www.septodontusa.com) was produced at Septodont using a free radical polymerizable resin developed by Jeffrey Stansbury and his team at the University of Colorado: this resin contains monomers derived from dimer acid.4 The dimer acid derivative monomers produce homopolymers with very high conversion, low shrinkage, and extreme hydrophobicity, avoiding water uptake. Their copolymerization with conventional monomers currently used in dental applications allows polymerization shrinkage much lower than would be anticipated based on the resin composition. To produce the low levels of polymerization shrinkage, a phase separation is required in the final polymer. For efficient photopolymerization, the phase separation is controlled to limit the degree of opacity and improve the physical properties. The resin system is non-phase separated prior to polymerization.5,6
In addition to this resin system, a new filler system was designed using nanotechnology. It is a combination of two types of nanofillers: silica and ytterbium fluoride and regular barium glass. The system using dimer acid derived monomers adopted for N’Durance achieves a monomer conversion of 83% (75% when fillers are added) with low volumetric contraction, polymerization stress, water uptake, and solubility.7
Objective
The study aimed to determine physical and chemical properties of N’Durance compared to the other principal products on the market.
Materials and Methods
Materials tested in this study are listed in Table 1. Specimens were polymerized following the manufacturers’ instructions, using a visible light lamp with an intensity of 500 mW/cm2.
Compressive and flexural strengths, flexural modulus, water sorption, and solubility were determined according to the International Organization for Standardization ISO 4049 and ADA Specification 27. Diametral tensile strength was mesured using a universal mechanical testing machine with a similar method as compressive strength. Shrinkage stress was measured with a tensometer (ADAHF, https://www.ada.org). Polymerization shrinkage was measured using a linometer from ACTA (Academisch Centrum Tandheelkunde Amsterdam, https://www.acta.nl). Monomer conversion was calculated by near infrared (IR) measuring the peak area corresponding to C = C bond before and after polymerization. The percentage of double bond conversion was calculated using the equation:
Results
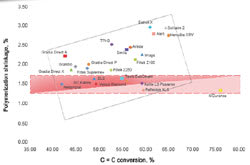
Table 2 summarizes results obtained on monomer conversion, volume shrinkage, and shrinkage stress of various available composite materials. It was shown that products using conventional resin, bisphenyl A glycidyl dimethacrylate (Bis-GMA)/triethylene glycol dimethacrylate (TEGDMA), have an average conversion of 60% for the microhybrids and 50% for the nanofilled composites. If the final double bond conversion is increased to reduce the unreacted monomers, the volumetric shrinkage and shrinkage stress also increase.
Even with the large monomer conversion, the shrinkage stress and contraction with N’Durance are lower than most commercial products. The shrinkage contraction was reduced from 2.3% (average of the regular composite) to 1.5%, and the shrinkage stress from 2.5% to 1.1%, with an increase of approximately 27% in the monomer conversion. This lower volumetric contraction can be explained by the phase separation that appears during polymerization of the new resin system, which produces an expansion that compensates for the volumetric shrinkage.7
Manufacturers battle the problem of an increase in shrinkage contraction and stress when the monomer conversion increases.8 This issue has been solved using the new resin system based on a dimer acid derivative monomer. As shown in Figure 1, none of the low-shrinkage products exhibits an elevated conversion degree as N’Durance does. The higher conversion can maintain greater strength (Table 3) and, with the hydrophobicity of the system, avoid water uptake/solubility (Table 4), decreasing the potential for unreacted monomers to be extracted from the composite. Thus, the greater conversion may increase the biocompatibility of this composite.
Conclusion
N’Durance, which uses a new resin system based on a dimer acid derivative monomer, shows higher monomer conversion without deteriorating other properties, such as low volume shrinkage, low shrinkage stress, and high strength. In addition, there is a decrease in the water uptake.
For products that use conventional resin Bis-GMA/ TEGDMA, volumetric shrinkage increases when the final double bond conversion is increased to reduce the unreacted monomers. N’Durance shows a unique response: high conversion and low polymerization shrinkage.
About the Authors
Cora Bracho-Troconis, PhD
R&D Director
Septodont
Colorado Research Center
Louisville, Colorado
Marianela Trujillo-Lemon, PhD
R&D Manager, Septodont
Colorado Research Center
Louisville, Colorado
Jordan Boulden, BSc
Septodont
Colorado Research Center
Louisville, Colorado
Nicolas Wong, BSc
Septodont
Colorado Research Center
Louisville, Colorado
Kristin Wall, BSc
Septodont
Colorado Research Center
Louisville, Colorado
Kristina Esquibel, BSc
Septodont
Colorado Research Center
Louisville, Colorado
References
1. Trujillo-Lemon M, Ge J, Lu H, Tanaka J, Stansbury JW. Dimethacrylate derivatives of dimer acid. J Polym Sci A Polym Chem. 2006;44(12):3921-3929.
2. Ge J, Trujillo-Lemon M, Lu H. Stansbury JW. Investigation of alternative dimethacrylate structures in dental resins [abstract]. ACS Polymer Preprints. 2005;46(2).
3. Tanaka J, Stansbury JW, Antonucci JM. New hydrophobic diluent monomers for UDMA and Bis-GMA. Paper presented at: the 78th General Session & Exhibition of the International Association for Dental Research; 2000; Washington DC.
4. Stansbury JW, Bowman CH, Trujillo M, inventors; Confi-Dental Products Co, assignee. Dimer acid-derived dimethacrylates and use in dental restorative compositions. US patent application 04815826.5-2318. December 29th, 2004.
5. Ge J, Trujillo-Lemon M, Lu H, Stansbury JW. Dimer acid-derived dimethacrylates as diluent monomers in restorative resins [abstract 1470]. J Dent Res. 2005(special issue A);84.
6. Stansbury JW. Modifying dental resins with monomers based on dimer acid [abstract 789]. J Dent Res. 2001(special issue A);80.
7. Bracho-Troconis CB. N’Durance® Product Scientific File. Louisville, CO: Septodont Confi-Dental Division; 2008.
8. Weir M, Richards N, Antonucci J. Structural effects of experimental comonomers on conversion and polymerization shrinkage of dental composites. Society for Biomaterials 29th Annual Meeting Transactions. 2003:543.